Arsenic acid, H3AsO4, is a triprotic acid with the following acid dissociation constants:
Ka1 = 5.5 × 10-3 ? Ka2 = 1.7 × 10-7 ? Ka3 = 5.1 × 10-12
Which of the following combinations would be best for preparing a pH 7 buffer?
A. Na2HAsO4 and Na3AsO4
B. H3AsO4 and HCl
C. H3AsO4 and Na3AsO4
D. NaH2AsO4 and Na2AsPO4
E. H3AsO4 and NaH2AsO4
Answers
The best combination for preparing a pH 7 buffer is option C, H3AsO4 and Na3AsO4.The option C is correct.
Arsenic acid, H3AsO4, is a triprotic acid with the following acid dissociation constants:Ka1 = 5.5 × 10-3 ? Ka2 = 1.7 × 10-7 ? Ka3 = 5.1 × 10-12. The best combination for preparing a pH 7 buffer is Na2HAsO4 and Na3AsO4.
What is a buffer?
A buffer is a substance that can neutralize an acid or a base to preserve a stable pH range.
A buffer is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is formed when an acid and its corresponding base or a weak acid and its conjugate base are combined in equal amounts. A buffer solution maintains its pH because the conjugate base of the weak acid neutralizes any added H+ ions
Whereas the weak acid neutralizes any added OH– ions. Thus, if the mixture contains both the weak acid and its conjugate base, the pH remains stable. Therefore, the best combination for preparing a pH 7 buffer is Na2HAsO4 and Na3AsO4.
To learn more about triprotic acid here:
https://brainly.com/question/29068526
#SPJ11
Related Questions
Identify the steps you would take to determine the percent by mass of water that makes up a sample of hydrated copper (II) sulfate (CuSO, 5H20).
Answers
To determine the percent by mass of water in a sample of hydrated copper (II) sulfate (CuSO₄ · 5H₂O), divide the molar mass of 5H₂O by the molar mass of CuSO₄ · 5H₂O and multiply by 100.
How do you determine the percent by mass of water in a sample of hydrated copper (II) sulfate (CuSO4 · 5H2O)?To determine the percent by mass of water in a sample of hydrated copper (II) sulfate (CuSO₄ · 5H₂O), you can follow these steps:
Determine the molar mass of CuSO₄ and H₂O:CuSO₄: Copper (Cu) has a molar mass of 63.55 g/mol, sulfur (S) has a molar mass of 32.07 g/mol, and oxygen (O) has a molar mass of 16.00 g/mol. So, the molar mass of CuSO4 is 63.55 + 32.07 + (4 × 16.00) = 159.61 g/mol.H₂O: Hydrogen (H) has a molar mass of 1.01 g/mol, and oxygen (O) has a molar mass of 16.00 g/mol. So, the molar mass of H₂O is 2 × 1.01 + 16.00 = 18.02 g/mol.Calculate the molar mass of CuSO₄ · 5H₂O:CuSO₄ · 5H₂O consists of 1 CuSO₄ unit and 5 H₂O units. Therefore, the molar mass of CuSO4 · 5H₂O is (1 × molar mass of CuSO₄) + (5 × molar mass of H₂O).Determine the molar mass of the water portion in CuSO₄ · 5H₂O:The molar mass of 5H₂O is 5 × molar mass of H₂O.Calculate the percent by mass of water:Divide the molar mass of the water portion by the molar mass of CuSO₄ · 5H₂O and multiply by 100 to get the percent.By following these steps, you can determine the percent by mass of water in a sample of hydrated copper (II) sulfate (CuSO₄ · 5H₂O).
Learn more about hydrated copper
brainly.com/question/21537790
#SPJ11
What is the activation energy for the reaction in this energy diagram?

Answers
Answer:
+ 100 Kj
Explanation:
The very minimum energy required to activate atoms or molecules to undergo chemical transformation.
Answer:
It is 60kj.
Explanation:
It is the highest point minus where a+b is at, so it is 60kj.
what influences whether a compound is insoluble, partially/weakly soluble, or fully soluble in water?
Answers
The solubility of a compound is influenced by factors such as temperature, pressure, concentration, and the nature of the solute and solvent.
It is critical to note that temperature and pressure have a greater impact on the solubility of solids and liquids than on gases. Let's explore each of these factors in more detail.
Temperature: The solubility of solid compounds in water generally increases as temperature increases. For example, sugar is more soluble in hot water than in cold water. The solubility of gas compounds in water decreases as temperature increases. As a result, it is harder to dissolve CO2 in warm soda than in cold soda.
Pressure: Pressure affects the solubility of gases. As the pressure increases, the solubility of a gas in a solvent increases as well. This is why soda can be carbonated at high pressures.
Concentration: The concentration of the solute in the solvent has an effect on the solubility of a compound. The solubility of a compound decreases as its concentration in the solvent increases. If you add more and more sugar to water, it will eventually become saturated, which means that it cannot dissolve any more sugar.
Nature of the solute and solvent: The solubility of a compound is also influenced by the nature of the solute and solvent. For example, polar solvents like water dissolve polar compounds, while nonpolar solvents dissolve nonpolar compounds. If the solute and solvent are polar, the solute is more likely to dissolve in the solvent.
Learn more about solute here:
https://brainly.com/question/30665317
#SPJ11
what is the atomic number for the following isotope: the chromium isotope with 28 neutrons.
Answers
The atomic number for the chromium isotope with 28 neutrons is 24, and the isotope is known as Chromium-52.
The atomic number of an element refers to the number of protons in its nucleus. Therefore, to determine the atomic number of the chromium isotope with 28 neutrons, we need to know the total number of particles in the nucleus. The atomic mass of chromium is 52, which is the sum of its protons and neutrons.
Since we know that this particular isotope has 28 neutrons, we can subtract that from the atomic mass to find the number of protons. 52 - 28 = 24. Therefore, the atomic number of the chromium isotope with 28 neutrons is 24. This means that the atom has 24 protons in its nucleus and 24 electrons surrounding the nucleus to maintain electrical neutrality.
More on isotopes: https://brainly.com/question/681602
#SPJ11
100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS 100 POINTS


Answers
Answer:
2.2 moles
Explanation:
n = CV
n = 0.4×5.50
n=2.2 moles
Answer:
Explanation:
#2 is answered so here is for #3:
Knowns: 100mL of solution; concentration of 0.7M
Unknown: number of moles
Equation: number of moles = volume * concentration
Plug and Chug: number of moles = 100/1000 * 0.7 = 0.07 mole
Final Answer: 0.07mole
If 22.00 J of energy is used, what would be the mass of liquid ammonia that could be vaporized? 1.38 kJ/g
Answers
Answer:
32kg/j correct me if I am wrong
Answer:
1.38 kJ/g
Explanation:
it is answer
what mass of methanol is produced when 280.2 g of carbon monoxide reacts with 50.5 g of hydrogen? CO(g)+2H2(g)—> CH3OH(l)
Answers
Answer:
320.23g of CH3OH.
Explanation:
We'll begin by writing the balanced equation for the reaction. This is given below:
CO(g) + 2H2(g)—> CH3OH(l)
Next, we shall determine the masses of CO and H2 that reacted and the mass of CH3OH produced from the balanced equation. This is illustrated below below:
Molar mass of CO = 12 + 16 = 28g/mol
Mass of CO from the balanced equation = 1 x 28 = 28g
Molar mass of H2 = 2x1 = 2g/mol
Mass of H2 from the balanced equation = 2 x 2= 4g
Molar mass of CH3OH = 12 + (3x1) + 16 + 1 = 32g/mol
Mass of CH3OH from the balanced equation = 1 x 32 = 32g
From the balanced equation above,
28g of CO reacted with 4g of H2 to produce 32g of CH3OH.
Next, we shall determine the the limiting reactant. This is illustrated below:
From the balanced equation above,
28g of CO reacted with 4g of H2.
280.2g of CO will react with =
(280.2 x 4)/28 = 40.03g of H2.
From the calculations made above, we can see that only 40.03g out of 50.5g of H2 is required to react completely with 280.2g of CO.
Therefore, CO is the limiting reactant and H2 is the excess reactant.
Finally, we shall determine the mass of methanol, CH3OH produced from the reaction.
In this case, the limiting reactant will be used because it will give the maximum yield of the reaction since all of it is used up in the reaction. The limiting reactant is CO and the mass of methanol, CH3OH produced can be obtained as follow:
From the balanced equation above,
28g of CO reacted to produce 32g of CH3OH.
Therefore, 280.2g of CO will react to produce = (280.2 x 32)/28 = 320.23g of CH3OH.
Therefore, 320.23g of CH3OH were produced from the reaction.
FILL IN THE BLANK. [ne]3s23p3 is the electron configuration of a(n) ________ atom.
Answers
Help would be greatly appreciated, picture of the question is here

Answers
Based on the results obtained in the table, the solute is a solid because it has an increase in the amount dissolved as temperature increases.
The correct option is B.
What is the effect of an increase in temperature on the solubility of solutes?The average kinetic energy of the solute molecules increases along with the solution's temperature. As a result, the molecules have a lower ability to bind together and dissolve more easily. Therefore, when the temperature rises, solid states become more soluble.
However, for solutes that are gas, an increase in temperature decreases the solubility of the gas, and the gas comes out of the solution.
Learn more about solubility and temperature at: https://brainly.com/question/23946616
#SPJ1
In rabbits, short fur (f) is dominant to long fur (1). what is the probability of two heterozygous rabbits having offspring with short fur?
25%
50%
75%
100%
Answers
50%
Because f is dominant to 1, it will be a 50/50 chance of the offspring having short fur, or long fur.
Pls correct me if im wrong
State Raoult’s law ?
Answers
Answer:
Established by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture.
Explanation:
you are trying to acidify a liquid in a breaker, but no matter how much hcl (strong acid) you dump in the liquid, the ph remains at 7. the liquid in the breaker can best be described as a
Answers
The given solution is a buffer.
When acidic or basic components are added to a buffer solution, it can withstand changes in pH. The solution's pH can be maintained relatively stable by neutralizing small amounts of added acids or bases. This is important for processes and reactions that require a specific and stable pH range. A buffer is an aqueous acid or base solution consisting of a mixture of a weak acid and its conjugate base or vice versa. Addition of a small amount of strong acid or strong base hardly changes the pH.
Learn more about buffer
brainly.com/question/13076037
#SPJ4
What volume of hydrogen sulfide gas will be collected if
39.0 grams of sodium sulfide are reacted?
Answers
Answer:
yes
Explanation:
Answer:
578.....882...767..86
can i have help with this please

Answers
An acid-base reaction occurs when a fatty acid interacts with NaOH, and the fatty acid transfers a proton to the base to produce sodium carboxylate salt and water.
What is saponification ?Triglycerides are converted into glycerol and "soap" when they are saponified, or reacted with sodium or potassium hydroxide (lye). The triglycerides are often made of vegetable or animal fats. A hard soap is created when sodium hydroxide is used.
Fats, oils, or lipids (the acid) are turned into soap by the process of saponification by mixing them with sodium hydroxide (the base). Friction and heat from the chemical reaction are essential. The neutralization of the acid and base occurs during saponification.
Thus, any type of fat and sodium hydroxide react chemically in a process known as saponification.
To learn more about saponification follow the link;
https://brainly.com/question/16495580
#SPJ1
How do the ramp heights of the different objects compare? How does the ramp height relate to the strength of the frictional force between the book and the object?
Answers
The height of a ramp does not directly determine the strength of the frictional force between a book and an object.
How do they compare?The strength of the frictional force between a book and an object is not directly influenced by the height of a ramp. The nature of the surfaces in contact, the force forcing the surfaces together (normal force), and the coefficient of friction are some of the variables that affect the frictional force between two surfaces.
The coefficient of friction between the book and the object plays a major role in determining the strength of the frictional force.
Learn more about frictional force:https://brainly.com/question/30280206
#SPJ1
Please help me figure this question out I’ll mark you brainiest. The balanced equation would be 2Na + 2Cl = 2NaCl. Hope that helps ya solve it.

Answers
Answer:
I think c.............
The dog has a mass of 57kg and the boy has a mass of 48 kg. Who has more kinetic energy?

Answers
Answer:
The Dog
Explanation:
The more mass something has the more kinetic energy it has in it.
9.The density of mercury is 13.6 g/mL.
What is the mass in kilograms of a 2 L
commercial flask of mercury?
Answers
Answer:
27.2 kg
Explanation:
13.6 * 2 L of commercial flask of mercury = 27.2 kg.
Hope this helps! Have a good day! :D
Suppose I have enough ingredients to make 25 cookies, but when I go to bake them, I only get 5. What percent yield did I obtain?
Answers
Answer:
Explanation:
What is the "theoretical yield" of cookies from each of the "reactants?"
Theor. yield from eggs
=
12
eggs
×
24 cookies
1
egg
=
288 cookies
Theor. yield from vanilla
=
24
tsp vanilla
×
24 cookies
1
tsp vanilla
=
576 cookies
Theor. yield from salt
=
82
tsp salt
×
24 cookies
0.5
tsp salt
=
3936 cookies
Theor. yield from baking soda
=
84
tsp baking soda
×
24 cookies
0.5
tsp baking soda
=
4032 cookies
Theor. yield from chocolate chips
=
3
cups chocolate chips
×
24 cookies
1.333
cups chocolate chips
=
54 cookies
Theor. yield from sugar
=
11
cups sugar
×
24 cookies
0.5
cup sugar
=
528 cookies
Theor. yield from brown sugar
=
4
cups brown sugar
×
24 cookies
0.5
cup brown sugar
=
192 cookies
Finally, "we leave it as an exercise for the student" to calculate that
Theor. yield from margarine = 72 cookies
b. Identify the limiting reactant
The limiting reactant is the amount of chocolate chips because they can produce the smallest amount of cookies.
c. Determine the theoretical yield
The theoretical yield is 54 cookies, because that is all you can get from the chocolate chips provided.
The percent yield obtained on baking the cookies is 20 % and the theoretical yield is 25 cookies.
What is percent yield?Percent yield is defined as the ratio of actual yield to the theoretical yield multiplied by 100. If the actual and theoretical yield are same then the percent yield is 100%.If actual yield is less than the theoretical yield then the percent yield is less than 100%.Reason of this condition arising is the incompletion of reaction or loss of sample during recovery process.
In cases where percent yield is over 100% it indicates that more sample is recovered than the predicted amount.This condition arises when there are other simultaneous reactions taking place leading to the formation of product. It can also arise if there is incomplete removal of impurities from the sample .
As cookies obtained is 5 it is observed yield while 25 is theoretical yield so the percent yield, 5/25×100=20%.
Learn more about percent yield, here:
https://brainly.com/question/12704041
#SPJ2
Identify the type of energy that is NOT chemical energy?
A. butane in a lighter B. ball rolling down the hill C. food D. gasoline in a car E. battery
Answers
B. ball rolling down the hill
The type of energy that is NOT chemical energy is:
B. ball rolling down the hill
Chemical energy refers to the energy stored in chemical substances, such as the energy released during a chemical reaction or the energy stored in the bonds of molecules.
Options A, C, D, and E all involve examples of chemical energy:
A. Butane in a lighter:
The combustion of butane releases chemical energy in the form of heat and light.
C. Food:
The energy stored in food molecules, such as carbohydrates, fats, and proteins, is converted into chemical energy during metabolism.
D. Gasoline in a car:
The combustion of gasoline in an engine releases chemical energy, which is converted into mechanical energy to power the car.
E. Battery:
Batteries store chemical energy that can be converted into electrical energy through chemical reactions.
In contrast, option B, a ball rolling down the hill, involves kinetic energy. Kinetic energy refers to the energy of an object due to its motion.
As the ball rolls downhill, its energy is in the form of kinetic energy, not chemical energy.
Learn more about chemical energy from this link:
https://brainly.com/question/775910
#SPJ11
when a coil of wire is connected to a batter, an electric current flows through it. True or False
Answers
Answer:
True i think.
Explanation:
A 2.3 kg object has 15 ) of kinetic energy. Calculate its speed.
O A. 0.12 m/s
OB. 1.1 m/s
O c. 2.4 m/s
OD. 3.6 m/s
Answers
Answer:
The answer is option DExplanation:
To find the speed given the kinetic energy and mass we use the formula
\(v = \sqrt{ \frac{2KE}{m} } \\ \)
where
m is the mass
v is the speed
From the question
KE = 15 J
m = 2.3 kg
We have
\(v = \sqrt{ \frac{2 \times 15}{2.3} } = \sqrt{ \frac{30}{2.3} } \\ = 3.61157559...\)
We have the final answer as
3.6 m/sHope this helps you
Part A. What are the possible values of the angular momentum quantum number l?
represented by the formula: l = k!, where k is a positive integer
represented by the formula: l = 2n + 1, where n is a principal quantum number
all the positive integers: 1,2,..., n-1, where n is a principal quantum number
all the positive integers in range from -n to +n, where n is a principal quantum number
all the integer: -3,-2,-1,0,1,2,3, etc.
represented by the formula: l = 2k + 1, where k is a positive integer
all the non-negative integers: 0,1,2,..., n-1, where n is a principal quantum number
Part B. What does the angular momentum quantum number determine?
Check all that apply.
the energy of an orbital
the orientation of the orbital
the energy of the electron on the outer shell
the possible number of electrons on particular orbital
the shape of the orbital
the overall size of an atom
the overall size of an orbital
Answers
Part A: The possible values of the angular momentum quantum number (l) are all the non-negative integers: 0, 1, 2, ..., n-1, where n is the principal quantum number.
In quantum mechanics, the angular momentum quantum number represents the magnitude of the angular momentum of an electron in an atom. Its values are determined by the principal quantum number (n) and determine the shape of the orbital. The formula l = 0, 1, 2, ..., n-1 indicates that the angular momentum quantum number can take on any non-negative integer value less than the principal quantum number.
Part B: The angular momentum quantum number (l) determines the shape of the orbital and the orientation of the orbital.
The angular momentum quantum number influences the spatial distribution and orientation of the electron cloud in an atom. It determines the shape of the orbital, such as s, p, d, or f orbitals. Additionally, it specifies the orientation or direction in space of the orbital within its designated shape. The other options listed (the energy of an orbital, the energy of the electron on the outer shell, the possible number of electrons on a particular orbital, the overall size of an atom, and the overall size of an orbital) are determined by different factors such as the principal quantum number, magnetic quantum number, and the total number of electrons in the atom.
learn more about "momentum ":- https://brainly.com/question/1042017
#SPJ11
What is physical since ?
Answers
Answer:
Phycical science is the study of the body.
Explanation:
The reaction between NO(g) and O2(g) produces a single product. The reaction occurs in a rigid reaction vessel represented in the diagram above. Which of the following statements correctly predicts the change in average molecular velocity of the molecules as the reaction goes to completion at constant temperature and provides the correct explanation
Answers
The average molecular velocity of the molecules will decrease as the reaction goes to completion at a constant temperature.
What is Molecular velocity?
Molecular velocity is the average speed of molecules in a sample of matter. It is determined by the temperature of the sample, with higher temperatures resulting in higher molecular velocities.
This is because the reaction produces a single product, meaning that the number of molecules in the reaction vessel is decreasing as the reaction goes to completion. As the number of molecules decreases, the average molecular velocity of the molecules decreases as well.
To know more about molecular velocity,
https://brainly.com/question/17031383
#SPJ4
what is the mass in grams of 0.00125 mole of sodium Na=23
Answers
Answer:
0.03g
Explanation:
Given parameters:
Number of moles of Na = 0.00125mole
Unknown:
Mass in grams of Na = ?
Solution:
To find the mass of this substance, we simply multiply the number of moles with the given molar mass of Na.
Mass of Na = number of moles x molar mass
Mass of Na = 0.00125 x 23 = 0.03g
Catalysts, such as the minerals deposited on hydrothermal vent walls, bring reactants together and __________.
Answers
Catalysts, such as the minerals deposited on hydrothermal vent walls, bring reactants together and increase the speed of the reactions without providing energy.
We can define catalysts as substances that increase the speed of a reaction. The catalysts themselves remain unchanged in a chemical reaction and work by lowering the temperature, pressure and energy levels.
Without a catalyst, a chemical reaction will take a long time to occur.
The rate of a reaction increases using a catalyst for the reaction. Some of the minerals that act as catalyst in hydrothermal vents are iron, nickel and sulfide minerals.
The oxides of most metals acts as catalysts. Inside the body of organisms, enzymes act as biological catalysts.
To learn more about catalysts, click here:
https://brainly.com/question/318426
#SPJ4
balancing chemical equations HELP BRO PLEASE
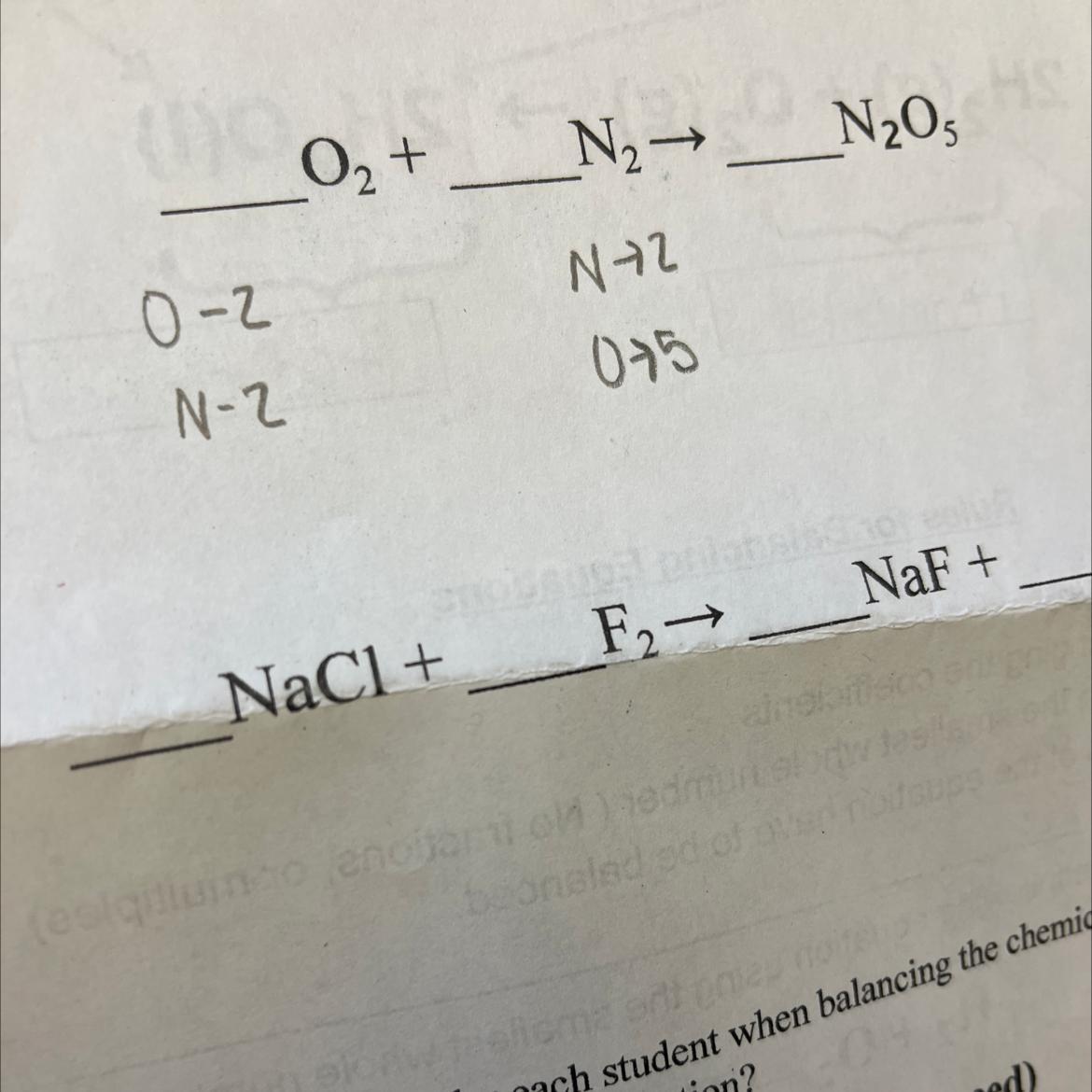
Answers
Answer:5O2 + 2N2 --> 2N2O5 and 2NaCl + F2 --> 2NaF + Cl2
Explanation:
1ST question
go to the products side and check the number of oxygen and nitrogen atoms , O-5 , N-2 , to balance this equation you need to add 2 in front of N2O5 and 5 in front of O2 (total oxygen atoms =10(5x2)). and 2 in front of N2 (total nitrogen atoms=4(2x2))
then : 5O2 + 2N2 --> 2N2O5
2nd question -
When NaCl (sodium chloride) reacts with fluorine the equation gives NaF(Sodium fluoride) so the remaining ion is chlorine so the other product needs to be chlorine gas which is Cl2
then : 2NaCl + 2F2 --> 2NaF + Cl2
nitrogen from a gaseous phase is to be diffused into pure iron at 675 c. if the surface concentraion is maintained at 2 wt% n, what will be the concentration 2 mm from the surface after 25 hours? the diffusion coefficient for nitrogen in iron at 675 c is 2.8 x 10^-11 m^2/s.
Answers
The concentration of nitrogen 2 mm from the surface after 25 hours is approximately 0.0198 wt%.
To find the concentration of nitrogen 2 mm from the surface after 25 hours, we can use Fick's second law of diffusion. The formula is given by:
C(x, t) = C0 * erfc((x / (2 * sqrt(D * t)))
Where:
C(x, t) is the concentration at a distance x from the surface at time t
C0 is the initial concentration at the surface
erfc is the complementary error function
D is the diffusion coefficient
x is the distance from the surface
t is the time
Given:
Initial concentration, C0 = 2 wt% = 0.02
Diffusion coefficient, D = 2.8 x 10^-11 m^2/s
Distance from the surface, x = 2 mm = 0.002 m
Time, t = 25 hours = 25 * 3600 seconds = 90000 seconds
Substituting these values into the formula:
C(0.002, 90000) = 0.02 * erfc((0.002 / (2 * sqrt(2.8 x 10^-11 * 90000))))
Evaluating this equation, we find that the concentration of nitrogen 2 mm from the surface after 25 hours is approximately 0.0198 wt%.
Learn more about concentration here :-
https://brainly.com/question/30862855
#SPJ11
A plastic ball with a volume of 19.7cm^3 has a mass of 15.8g Would this ball sink or float in a container of gasoline?
Answers
Answer:
It would sink because the density of the ball, 0.802 g/cm3, is greater than the density of gasoline.
On comparing the densities of both ball and gasoline, the density of the plastic ball is larger than that of petrol, it would sink in a container of petrol.
Mass is a physical property of matter that measures the amount of substance in an object. It is typically measured in grams (g) or kilograms (kg).
The density of the plastic ball can be calculated using the formula:
Density = Mass / Volume
Density of the plastic ball = 15.8 g / 19.7 \(\rm cm^3\) = 0.801 g/ \(\rm cm^3\)
The density of gasoline is about 0.7 g/ \(\rm cm^3\)
Since, the density of the plastic ball is greater than the density of gasoline, the plastic ball would sink in a container of gasoline.
Learn more about mass here:
https://brainly.com/question/11954533
#SPJ6