Arrange the following elements in order of decreasing metallic character: Rb, N, Si, P. Zn, and Al. Rank elements from most to least metallic character. Al Rb Si Zn N P Most metallic character Least metallic character The correct ranking cannot be determined.
Answers
The correct ranking of decreasing metallic character is Rb > Al > Si > Zn > N > P.
To determine the order of decreasing metallic character among the given elements, we need to consider their position in the periodic table.
Metals generally exhibit characteristics such as high electrical conductivity, luster, malleability, and ductility. Nonmetals, on the other hand, tend to have opposite properties.
Among the given elements, Rb (rubidium) is the most metallic since it is an alkali metal located in Group 1 of the periodic table. Al (aluminum) is also a metal, but it is less metallic than Rb.
Si (silicon), Zn (zinc), and N (nitrogen) are nonmetals, with Si being the least nonmetallic among them.
P (phosphorus) is also a nonmetal, and it is generally less metallic than N.
Based on this analysis, the correct ranking of decreasing metallic character is Rb > Al > Si > Zn > N > P.
To know more about periodic table, refer here:
https://brainly.com/question/11155928#
#SPJ11
Related Questions
Explain the difference between qualitative and quantitative properties.
Answers
Please help
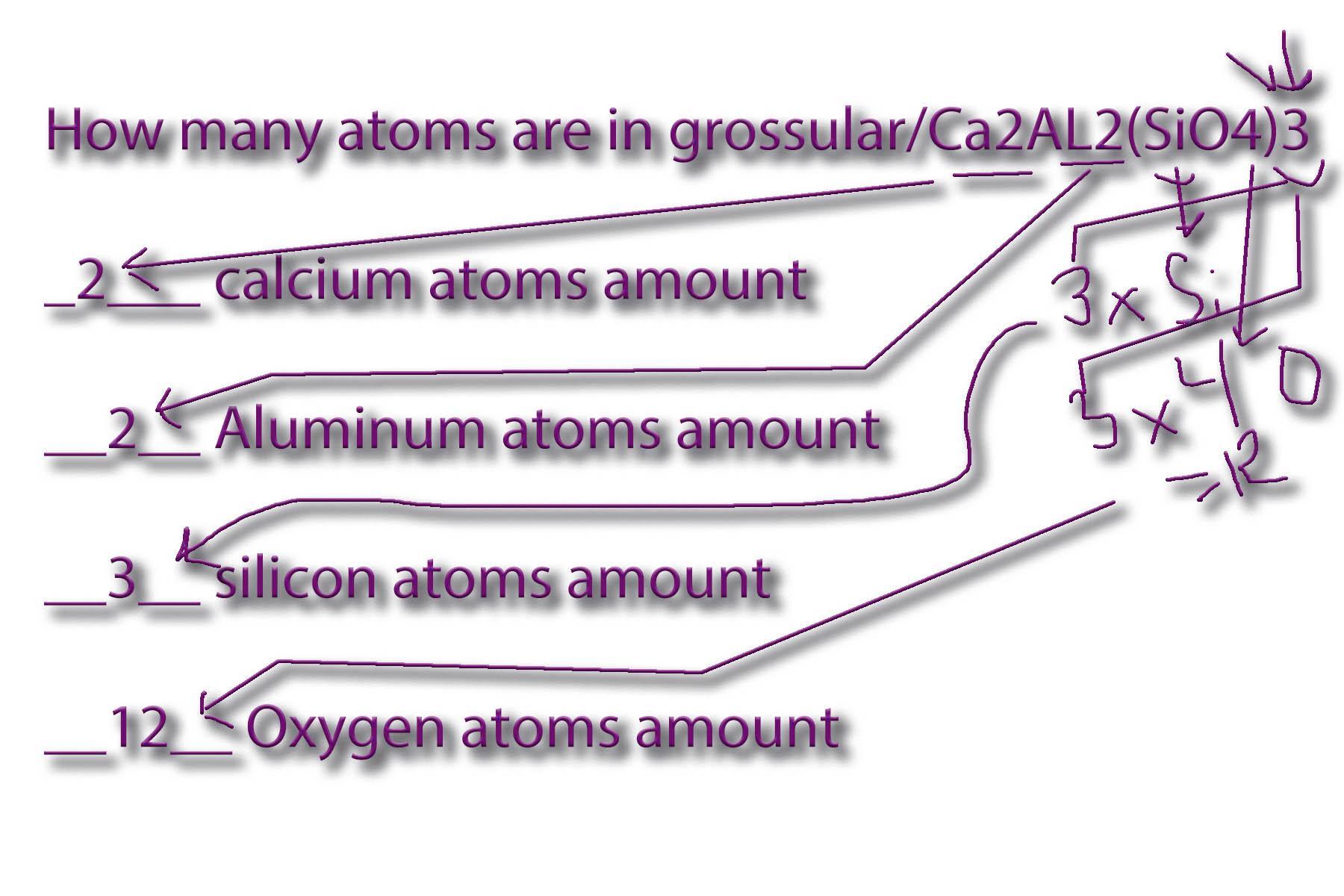
How many atoms are in grossular/Ca2AL2(SiO4)3
____ calcium atoms amount
____ Aluminum atoms amount
____ silicon atoms amount
____ Oxygen atoms amount
Answers
Answer:
See below
Explanation:
How many atoms are in grossular/Ca2AL2(SiO4)3
_2___ calcium atoms amount
__2__ Aluminum atoms amount
__3__ silicon atoms amount
__12__ Oxygen atoms amount
See attached worksheet
Engineers design cars with crumple zones to protect people inside cars they also added seat belts and air bags to protect people select words that describe how swag belts and air bags protect people during collisions
Answers
When it comes to car safety, engineers have implemented various measures to protect people during collisions. One of the primary safety features in modern cars is the seat belt. Seat belts work by preventing passengers from being thrown out of the vehicle in the event of a crash. They also reduce the impact of a collision by distributing the force of the impact across a larger surface area of the body. This reduces the risk of serious injuries or fatalities.
Airbags are another critical safety feature that works in conjunction with seat belts. Airbags deploy when a collision occurs, creating a cushion between the passenger and the interior of the car. This cushioning effect helps to reduce the force of impact and prevent injuries to the head and chest. Airbags also help to keep passengers from being ejected from the vehicle. In addition to seat belts and airbags, engineers design cars with crumple zones. Crumple zones are areas of the car designed to deform and absorb energy during a crash. This helps to reduce the impact of the collision and protect passengers inside the car.
Overall, seat belts, airbags, and crumple zones work together to provide comprehensive protection for passengers during collisions. By reducing the force of impact and preventing ejection from the vehicle, these safety features have saved countless lives on the road.
To know more about engineers visit:-
https://brainly.com/question/31140236
#SPJ11
which electrons of an atom take part in a bond formation
Answers
Answer:
The electrons in the outermost electron shell are responsible for forming chemical bonds. These electrons are also known as valence electrons. Chemical bonds are formed when the outermost electron shells are not completely filled and atom needs more (or less) electrons to completely fill the outermost shell.
Explanation:
Intermolecular forces define the boiling points of compounds. What type of intermolecular forces exist for Compounds 1, 2, and 3
Answers
Intermolecular forces are the forces that exist between molecules. The boiling point of a substance is a measure of its intermolecular forces.
If the intermolecular forces between the molecules in a substance are strong, then the boiling point will be high. There are three main types of intermolecular forces:Van der Waals forces Dipole-dipole interactions Hydrogen bonding Compounds 1, 2, and 3 are not provided in the question. Therefore, we cannot determine what type of intermolecular forces exist for these compounds. However, we can determine the intermolecular forces in a given compound by examining its molecular structure and composition.
Van der Waals forces are weak forces that exist between all molecules. These forces are caused by temporary dipoles that exist within molecules due to the random motion of their electrons. The strength of van der Waals forces depends on the size of the molecules. Larger molecules have more electrons and are therefore more polarizable, which leads to stronger van der Waals forces. Dipole-dipole interactions are forces that exist between polar molecules. These forces are caused by the attraction between the partial positive and partial negative charges on adjacent molecules. The strength of dipole-dipole interactions depends on the polarity of the molecules. Highly polar molecules have stronger dipole-dipole interactions. Hydrogen bonding is a special type of dipole-dipole interaction that occurs between molecules that have a hydrogen atom bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. This type of bonding is particularly strong and leads to high boiling points.
In conclusion, the boiling points of compounds are determined by the strength of the intermolecular forces between their molecules. Van der Waals forces are the weakest of the three types of intermolecular forces, followed by dipole-dipole interactions, and then hydrogen bonding. The strength of these forces depends on the size and polarity of the molecules and the presence of hydrogen bonding.
To know more about Hydrogen bonding visit:
brainly.com/question/31139478?
#SPJ11
Which stars have the highest absolute brightness?
The HR diagram is shown with Absolute Brightness on the y axis from negative four to six and Surface Temperature on the x axis from 40,000 to 2,500 degrees Celsius. There are several dots plotted between four and six absolute brightness and 10,000 to 2,500 degrees Celsius labeled Supergiants. There are several dots plotted between 1.5 and three absolute brightness and 7,500 to 2,500 degrees Celsius labeled Giants. There are several dots plotted between negative four and negative two absolute brightness and 30,000 to 7,500 degrees Celsius labeled Dwarfs. Down the middle, there are several dots plotted diagonally from ordered pair 40,000 and five down to 2,500 and negative three labeled Main Sequence.
Supergiants
Giants
Yellow stars
Dwarfs
Answers
Answer:Super-giants
Explanation:
What mass of KNO3 would have to be decomposed to produce 21.1 L of oxygen measured at STP?
2KNO3(s) −→ 2KNO2(s) + O2(g)
1. 202 g
2. 95.2 g
3. 190 g 4. 130 g
Answers
The mass of KNO3 required to produce 21.1 L of oxygen measured at STP is 95.2 g.
In the given balanced chemical equation, 2 moles of KNO3 produce 1 mole of O2. The molar volume of any gas at STP is 22.4 L/mol. To determine the mass of KNO3 needed, we first calculate the number of moles of O2 in 21.1 L using the ideal gas law: n = V/22.4. Thus, n = 21.1/22.4 = 0.941 moles of O2. Since the ratio in the balanced equation is 2:1 for KNO3 to O2, we need twice the number of moles of KNO3. Therefore, 0.941 moles of O2 would require 2 * 0.941 moles of KNO3, which is 1.882 moles. Finally, we determine the mass of KNO3 using its molar mass. The molar mass of KNO3 is approximately 101.1 g/mol. Thus, the mass of KNO3 needed is 1.882 moles * 101.1 g/mol = 190 g. Therefore, the correct answer is option 3: 190 g.
Learn more about KNO3 here ; brainly.com/question/14372543
#SPJ11
which explains how the nervous system is typically involved in keeping the body in Homeostasis?

Answers
Answer:
c because this is the one hundred all the time
Explanation:

Name the following covalent molecules:
SeF
Answers
Answer:
do you mean DeF?
Explanation:
4P + 5O2 → 2P2O5
What are the reactants in this chemical equation?

Answers
Answer:
P and O₂Explanation:
Greetings!!!
ReactantsThe substances which participate in a chemical reaction, are called reactants. A chemical reaction describes the process by which atoms, the fundamental building blocks of matter, rearrange themselves to form new combinations. Reactants are raw materials that react with one another.
Also, The substance(s) to the left of the arrow in a chemical equation are called reactants.
Hope it helps!!!
if the [H+] = 0.01 M, what is the pH of the solution, and is the solution a strong acid, weak acid, strong base, or weak base?
12, strong base
2, weak acid
12, weak base
2, strong acid
Answers
Answer:
2, strong acid
Explanation:
Data obtained from the question. This includes:
[H+] = 0.01 M
pH =?
pH of a solution can be obtained by using the following formula:
pH = –Log [H+]
pH = –Log 0.01
pH = 2
The pH of a solution ranging between 0 and 6 is declared to be an acid solution. The smaller the pH value, the stronger the acid.
Since the pH of the above solution is 2, it means the solution is a strong acid.
NEED ASAP! Will give brainliest to first answer

Answers
The following are matter properties and changes:
masssubstancepropertiesPhysicaldensitychemicalChemicalChemicalPhysicalPhysicalPhysicalChemicalPhysicalPhysicalPhysical (or Chemical, depending on the substance)PhysicalHow does matter change?Matter can change in a variety of ways, including physical and chemical changes. Physical changes involve changes in the physical properties of matter, such as its shape, size, and state (solid, liquid, or gas). These changes do not result in a change in the fundamental composition of the matter. Examples of physical changes include melting of ice, boiling of water, and bending of a metal rod.
Chemical changes involve changes in the chemical composition of matter, resulting in the formation of new substances with different chemical properties. Chemical changes involve the breaking and formation of chemical bonds between atoms or molecules. Examples of chemical changes include burning of wood, rusting of iron, and digestion of food.
Learn more on matter here: https://brainly.com/question/3998772
#SPJ1
The question is:
Matter-Properties and Changes
Use each of the terms below just once to complete the passage.
chemical density
Section 3.1 Properties of Matter
mass
properties
physical
substance
chemical
density
Matter is anything with (1)___________ and volume. A (2)___________ is a form of matter with a uniform and unchanging composition.
Substances have specific, unchanging (3)___________ that can be observed.
Substances have both physical and chemical properties. (4)___________ properties can be observed without changing a substance's chemical composition. Color, hardness, and (5)___________ are examples. Other properties cannot be observed without changing the composition of a substance. These are called (6)___________ properties. An example is the tendency of iron to form rust when exposed to air.
Label each property as either physical or chemical.
7. Chemical formula H₂O
8. Forms green carbonate when exposed to moist air
9. Remains unchanged when in the presence of nitrogen
10. Colorless
11. Solid at normal temperatures and pressures
12. Ability to combine with another substance
14. Liquid at normal temperatures and pressures
15. Boiling point is 100°C
16. Conducts electricity
17. Density is 1g/cm
A hot water bottle is a rubber container that can be filled with hot water and then sealed. What happens to the heat energy in the bottle when the bottle is placed on a pillow?.
Answers
The heat energy in the hot water bottle gets transferred to the pillow when it is placed on it.
When a hot water bottle is placed on a pillow, the heat energy inside it is transferred to the pillow. This is because the pillow has a lower temperature compared to the hot water bottle. Heat energy always flows from hot objects to colder ones until the two objects have the same temperature. This is a fundamental principle of thermodynamics called the second law of thermodynamics.
The transfer of heat energy occurs through a process called conduction, which is the transfer of heat through a material or from one object to another through direct contact. The rubber material of the hot water bottle and the pillow are both good conductors of heat, allowing for efficient transfer of heat between them.
Overall, the hot water bottle provides a source of heat, and when placed on a pillow, the heat energy is transferred from the hot water bottle to the pillow, making the pillow warmer.
Learn more about thermodynamics here:
https://brainly.com/question/1368306
#SPJ11
For the following calculations SHOW ALL WORK!! (6 points each)
7. The half- life of molybdenum-99 is 67.0 hours. How much of a 1.000 gram sample
remains after 335 hours.
8. Determine the average atomic mass of gallium if 60.00% of its atoms have a mass of
68.926 amu and 40.00% have a mass of 70.925 amu.
Answers
Answer:
60%
Explanation:
it is enough trust me
Name the apparatus that could be used to measure 50 cm³ of liquid hydrocarbon.
Answers
Answer: (ii) measuring cylinder / pipette / burette
Explanation:
Discuss the difference between renewable and non renewable resource and give and example of each
Will get brainliest for the best example!!!!!!!!!!!!!!!!!
Answers
Answer:
Nonrenewable energy resources, like coal, nuclear, oil, and natural gas, are available in limited supplies. ... Renewable resources are replenished naturally and over relatively short periods of time. The five major renewable energy resources are solar, wind, water (hydro), biomass, and geothermal.
Explanation:
Answer:
I am answering so the other guy can get brainliest: Renewable sources are sources that are *near-infinite (because the sun will eventually die one day) that are basically not depleted when used. Nonrenewable sources are sources that deplete when you use them, which are not easily replaceable. An example of a renewable resource is Solar Energy or Hydropower, a non-renewable source is fossil fuels or natural gas.
Explanation:
From the following items, which is closest in size to one mole of gas at stp?
Answers
At STP, a mole of pure gas is closest in size to a marble. As a tiny, solid material, a gram of gas at the STP is considerably smaller than a marble. D is the correct response.
At Standard Pressure and Temperature, 22.4 L of any gas will be required to hold 1 mole (STP). The Ideal Gas Law and a balanced chemical equation can be used to determine the amount or mass of gas consumed or created in a chemical process. In other words, the gas that has the greatest number of molecules of a certain gas at a given temperature will occupy the largest volume.
Learn more about STP
brainly.com/question/29356493
#SPJ4
Question: From the following items, which is closest in size to one mole of gas at STP?
A) A car
B) An elephant
C) A microwave
D) A marble
why does hydrogen fluoride have a high boiling point
Answers
The intermolecular bonding for HF is van der Waals, whereas for HCL, the intermolecular bonding is hydrogen. Since the van der Waals bond is stronger than hydrogen, HF will have a higher boiling temperature. Since the covalent bond is stronger than van der Waals, HF will have a higher boiling temperature.
6. How many grams of barium chloride are produced when 1.25 x 10power23 molecules of HCl reacts with barium according to the following equation?
Answers
word equation: HCl + barium --> barium chloride + hydrogen
formula equation: 2HCl + Ba --> BaCl2 + H2
given: 1.25×10^23 molecules HCl
Find: g BaCl2
given#moleculesSubA × 1molSubA/6.022×10^23 moleculeSubA × #molSubB/#molSubA × g SubB/1molSubB
The mass of barium chloride produced is 21.6 grams.
What is barium chloride?It is an inorganic compound with the chemical formula BaCl₂.
The equation: HCl + barium --> barium chloride + hydrogen
The chemical equation : 2HCl + Ba --> BaCl₂ + H₂
Given the 1.25×10²³ molecules HCl
First, we calculate the amount of HCL
Amount of moles of HCl=
(1.25×10²³ molecules × 1 mole)/ 6.023×10²³ atoms
=0.2075 moles.
Then the number of moles is multiplied by the molar mass of barium chloride
208.24 g/mol × 0.2075 = 21.6 grams.
Thus, the mass of barium chloride produced is 21.6 grams.
Learn more about barium chloride, here:
https://brainly.com/question/15296925
Express 0.044 km in meters.
Answers
Answer:
44
Explanation:
You multiply the length value by 1000
Answer:
0.044 km × 1000 = 44 meters
Which statement best describes the atoms in a gas?
Answers
Answer:
They move freely in all directions.
Explanation:
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
Determine the number of grams in 0.5 moles of H2 S04
Answers
Balancing Chemical Equations
I really need help

Answers
Answer:
2KClO3 ---2KCl + 3O2
2NaCl + F2 ----> 2NaF + Cl2
2AlBr3 + 3 K2SO4 ----> 6 KBr + Al2(SO4)3
An unknown liquid occupies a volume of 5 ml and has a mass of 40 grams. What is it's density?
Answers
Answer:
8000 kilogram/cubic meter
Explanation:
I used an online calculator.
Hope I helped!
Identify the molecular geometry corresponding to each expected bond angle around the central atom.
a. Linear b. Trigonal pyramidal c. Trigonal planar d. Tetrahedral
Answers
In Linear molecular geometry, the bond angle is 90°, in trigonal pyramidal geometry, bond angle is 107°, in trigonal planar geometry, bond angle is 120° and in tetrahedral, the bond angle is 109.5°.
In the linear geometry, the central atom has two side atoms attached which are at and bond angle of 180°.
In trigonal pyramidal geometry, the central atom has four side atoms which resembles a pyramid like structure. The bond angle between the two consecutive side atoms is 107°.
In trigonal planar geometry, three atoms are attached on the sides of central atom. The bond angle between these side atom is equal and of 120°.
In Tetrahedral geometry, the central atom and the side atoms makes a triangular prism like structure, the bond angle between side atoms is 109.5°.
To know more about Molecular Geometry, visit,
https://brainly.com/question/19354582
#SPJ4
how much heat is produced if 22.2 g of octane (c8h18) is combusted according to the following reaction? 2 c8h18 (g) 25 o2 (g) --> 16 co2 (g) 18 h2o (l) δh = -5471 kj/mol
Answers
If 22.2 g of octane (c8h18) is combusted according to the reaction, then the heat produced is -530.8 kJ.
Determining heat produced:
To determine how much heat is produced when 22.2 g of octane is combusted, follow these steps:
1. First, find the molar mass of octane (C8H18). For C (carbon), it's 12.01 g/mol, and for H (hydrogen), it's 1.01 g/mol.
Molar mass of C8H18 = (8 x 12.01) + (18 x 1.01) = 96.08 + 18.18 = 114.26 g/mol.
2. Calculate the number of moles of octane combusted.
Moles of octane = mass of octane / molar mass of octane = 22.2 g / 114.26 g/mol = 0.194 mol.
3. Determine the ratio of moles of octane in the balanced chemical equation.
According to the balanced equation, 2 moles of octane release -5471 kJ of heat energy.
4. Calculate the heat produced for the given moles of octane.
Heat produced = (0.194 mol octane / 2 mol octane) x (-5471 kJ) = 0.097 x (-5471 kJ) = -530.8 kJ.
So, when 22.2 g of octane is combusted, -530.8 kJ of heat is produced.
To know more about the combustion, visit: https://brainly.com/question/15117038
#SPJ11
which element would be expected to have properties similar to argon? group of answer choices h br kr cl
Answers
Krypton is the element that would be expected to have properties similar to argon, while hydrogen, chlorine, and bromine cannot be expected to have similar properties due to their incomplete outer electron shells.
Argon is a noble gas and belongs to Group 18 (also called Group 8A or Group 0) of the periodic table. Elements in this group have a full outer electron shell, making them very stable and unreactive. Therefore, an element with similar properties to argon would also need to have a full outer electron shell.
Hydrogen (H) and chlorine (Cl) do not have a full outer electron shell and are highly reactive. Therefore, they cannot be expected to have properties similar to argon.
Bromine (Br) is a halogen and belongs to Group 17 (also called Group 7A) of the periodic table. Elements in this group have one less electron than Group 18 elements, so they are highly reactive and form compounds readily. Therefore, bromine cannot be expected to have properties similar to argon.
Krypton (Kr), on the other hand, is also a noble gas and belongs to Group 18 like argon. Like argon, krypton has a full outer electron shell, making it stable and unreactive. Therefore, krypton can be expected to have properties similar to argon. Both argon and krypton are inert and have low boiling and melting points due to weak van der Waals forces between their atoms.
To know more about Properties of element:
https://brainly.com/question/30388913
#SPJ4
Predict whether or not the substances in the table will sublime at STP. Base your predictions only on the type of force holding the solid together.

Answers
The states of matter of the materials;
1) Dispersion forces - Yes
2) Hydrogen bonding - No
3) Ionic - No
4) Dispersion forces - No
5) Dispersion forces - Yes
6) Ionic - No
7) Hydrogen bonding - No
What is the sublimation?
Sublimation is a physical process in which a substance transitions directly from its solid phase to its gaseous phase without passing through the intermediate liquid phase. In sublimation, the solid substance is heated, and the resulting gas molecules escape from the solid lattice structure without the need for melting.
One common example of sublimation is the process of dry ice. Dry ice is solid carbon dioxide (CO2) that sublimes at a temperature of -78.5 degrees Celsius (-109.3 degrees Fahrenheit). When dry ice is exposed to room temperature, it transitions directly from a solid to a gas, producing a fog-like effect.
Learn more about states of matter:https://brainly.com/question/9402776
#SPJ1
This is the chemical formula for the methyl tert-butyl and ether (the clean-fuel gasoline additive MTBE): Ch3OC(CH3)3
A chemical engineer has determined by measurements that there are 0.0946 moles of carbon in a sample of methyl tert-butyl ether. How many moles of oxygen are in the sample? Be sure your answer has the correct number of significant digits.
Answers
The sample of methyl tert-butyl ether has 0.0946 moles of oxygen.
When calculating the amount of oxygen in a sample of methyl tert-butyl ether (MTBE), it is important to take into account the chemical formula \(CH_{3}OC(CH_{3})_{3}.\)
Each molecule of MTBE has one oxygen atom (O), as can be seen from the formula. The sample has 0.0946 moles of carbon (C), according to the information provided.
Since MTBE has a carbon to oxygen content of 1:1, there will be 0.0946 moles of oxygen in total.
Therefore, the sample of methyl tert-butyl ether has 0.0946 moles of oxygen.
Learn more about moles, here:
https://brainly.com/question/30885025
#SPJ1