Answers
The activation energy is 50kJ
The enthalpy change of the reaction is -20kJ
The reaction is exothermic
B is the products while A is the reactants
What is the activation energy?The activation energy is the very minimum of energy needed for a chemical reaction to take place.
In other words, for the reaction to proceed, the reactants must have sufficient energy to pass the energy barrier or activation energy.
This energy is needed to release the bonds between the reactants and start the reaction.
Learn more about activation energy:https://brainly.com/question/28384644
#SPJ1
Related Questions
Pls help with science

Answers
A reaction is expected to produce 28. 3 moles of hydrogen gas. If the hydrogen is collected at 297 K and 1. 08 atm, what is the volume? 305 L H2 639 L H2 948 L H2 1,240 L H2.
Answers
To find the volume of hydrogen gas, we can use the ideal gas law equation: PV = nRT. Rearranging the equation to solve for V (volume), we have V = (nRT) / P. Given that n = 28.3 moles, R is the ideal gas constant, T is 297 K, and P is 1.08 atm, we can substitute these values to find the volume.
To determine the volume of hydrogen gas produced, we can use the ideal gas law equation, which states:
PV = nRT
Where:
P = pressure (in atm)
V = volume (in liters)
n = moles of gas
R = ideal gas constant (0.0821 L·atm/(mol·K))
T = temperature (in Kelvin)
Given:
n = 28.3 moles
T = 297 K
P = 1.08 atm
Substituting the values into the equation, we can solve for V:
V = (nRT) / P
V = (28.3 moles * 0.0821 L·atm/(mol·K) * 297 K) / 1.08 atm
V ≈ 948 L
Therefore, the volume of hydrogen gas produced is approximately 948 L.
To know more about ideal gas law click this link -
brainly.com/question/12624936
#SPJ11
A given volume of methane diffuses in 20seconds. How long will it take the same volume of sulphur(4)oxide to diffuse under the same condition?
Answers
40 seconds long it take the same volume SO₂ to diffuse under the same condition.
Given that,
A volume of methane diffuses in 20 seconds.
We have to find how long will it take the same volume of SO₂ to diffuse under the same condition when CH₄ = 16 and SO₂ = 64
We know that,
What is Graham's law?Graham's law state that inversely proportional to the square root of its molecular mass is equal to the rate of effusion of a gas.
So,
By applying the Graham's law,
\(\frac{r_1}{r_2} =\sqrt{\frac{M_2}{M_1} }\)
\(\frac{r(SO_2)}{r(CH_4)} =\sqrt{\frac{M(CH_4)}{M(SO_2)} } = \frac{t(CH_4)}{t(SO_2)}\)
Where t(SO₂) = x, M(SO₂) = 64
t(Ch₄) = 20 sec, M(Ch₄) = 16
So,
\(\frac{t(SO_4)}{20} = \sqrt{\frac{64}{16} }\)
t(SO₄) = 20 × \(\frac{8}{4}\)
t(SO₄) = 40 seconds.
Therefore. 40 seconds long it take the same volume SO₂ to diffuse under the same condition.
To know more about volume visit:
https://brainly.com/question/31435586
https://brainly.com/question/30178583
(60 points + brain list)
Which is the configuration of an atom in the ground state?
A)
2-8-2
B)
2-6-1
C)
2-7-2
D)
2-7-3
Answers
Answer:
Ground state means when n = 1
and here the electronic configuration is 2:8:2
what is the standard form of 12,341 us?
Answers
Answer:
12341 in standard form = 1.2341 × 10⁴
Explanation:
When you split the number 12341 into a coefficient and a power of 10 you do get 12341 in exponential form, but there is an indefinite number of possibilities.
As you probably want 12341 in normalized scientific notation, the coefficient or significand of 12341 in scientific notation must be in the interval [1,10[.
As there are many ways to express 12341 in scientific notation, in this post we mean 12341 in normalized scientific notation, unless stated otherwise. Therefore:
12341 in scientific notation = 1.2341 × 10⁴.
This can also be expressed as 1.2341 × 10⁴, using the caret symbol, or as 1.2341e+4, which is called 12341 in e-notation.
0.884 mol sample of methane gas at a temperature of 6.00 C is found to occupy a volume of 26.5 L. The pressure of this gas sample is ____ mm Hg.
Answers
Answer:
580mmHg
Explanation:
Given parameters:
Number of moles = 0.884 mol
Temperature = 6°C = 273 + 6 = 279k
Volume = 26.5L
Unknown:
Pressure = ?
Solution:
To solve this problem, we use the ideal gas equation:
PV = nRT
P is the pressure
V is the volume
n is the number of moles
R is the gas constant = 0.082atmdm³mol⁻¹K⁻¹
T is the temperature
P = \(\frac{nRT}{V}\) = \(\frac{0.884 x 0.082 x 279}{26.5}\) = 0.76atm
1 atm = 760mmHg
0.76 atm = 760 x 0.76 = 580mmHg
Consider a buffer solution that is 0. 50 M in NH3 and 0. 20 M in NH4Cl. For ammonia, pKb=4. 75. Calculate the pH of 1. 0 L of the solution upon addition of 30. 0 mL of 1. 0 M HCl to the original buffer solution.
Express your answer to two decimal places
Answers
The pH of 1. 0 L of the solution on addition of 30. 0 mL of 1. 0 M HCl to the original buffer solution will be 12.50.
The reaction that occurs when HCl is added to the buffer solution is:
HCl + NH₃ → NH₄⁺ + Cl⁻
The HCl reacts with NH₃ to form NH₄⁺ and Cl⁻. This will cause the concentration of NH₄⁺ in the buffer to increase and the concentration of NH₃ to decrease. However, since we started with a buffer solution, it will still be able to resist changes in pH.
To solve this problem, we will use the Henderson-Hasselbalch equation:
pH = pKb + log([NH₄⁺]/[NH₃])
where [NH₄⁺] is the concentration of the ammonium ion and [NH3] is the concentration of ammonia.
Calculate the moles of HCl added
The volume of HCl added is 30.0 mL = 0.0300 L. The concentration of HCl is 1.0 M, so the moles of HCl added are:
moles of HCl = concentration x volume = 1.0 M x 0.0300 L = 0.0300 moles
Calculate the new concentrations of NH₄⁺ and NH₃
The moles of NH₄⁺ and NH₃ in the original buffer solution can be calculated as:
moles of NH₄⁺ = 0.20 M x 1.0 L = 0.20 moles
moles of NH₃ = 0.50 M x 1.0 L = 0.50 moles
When HCl is added, it reacts with NH₃ to form NH₄⁺ and Cl⁻. The amount of NH₄⁺ produced is equal to the amount of HCl added, since the reaction is 1:1. Therefore, the new concentration of NH₄⁺ is:
[NH₄⁺] = moles of NH₄⁺ / (volume of buffer + volume of HCl added)
[NH₄⁺] = 0.20 moles / (1.0 L + 0.0300 L)
[NH₄⁺] = 0.196 M
The new concentration of NH₃ can be calculated using the buffer equation:
[NH₃] = Ka x [NH₄⁺] / [H⁺]
where Ka is the equilibrium constant for the reaction NH₄⁺ + H₂O → NH₃ + H₃O⁺, which is equal to the acid dissociation constant of NH₃, Kb. Since pKb is given as 4.75, we can calculate Kb:
Kb = 10^(-pKb) = \(10^{-4.75}\) = 1.78 x 10⁻⁵
Substituting the values we have:
[NH3] = Kb x [NH₄⁺] / [H⁺]
[NH3] = 1.78 x 10⁻⁵ x 0.196 M / \(10^{-pH}\)
[NH3] = 3.49 x 10⁻⁶ / \(10^{-pH}\)
Calculate the new pH of the buffer
Substituting the values we have into the Henderson-Hasselbalch equation:
pH = pKb + log([NH₄⁺]/[NH₃])
pH = 4.75 + log(0.196 M / (3.49 x 10⁻⁶ / \(10^{-pH}\))))
Simplifying and solving for pH:
pH = 4.75 + log(5.61 x 10⁷) + log(\(10^{pH}\))
pH = 4.75 + 7.75 + pH
pH = 12.50
To know more about buffer solution here
https://brainly.com/question/15709146
#SPJ4
What is patagium and what do you meant by prehensile tail
Answers
The patagium (plural: patagia) is a membranous structure that assists an animal in gliding or flight. The structure is found in living and extinct groups of animals including bats, birds, some dromaeosaurs, pterosaurs, gliding mammals, some flying lizards, and flying frogs.
A prehensile tail is the tail of an animal that has adapted to grasp or hold objects. Fully prehensile tails can be used to hold and manipulate objects, and in particular to aid arboreal creatures in finding and eating food in the trees.
Answer:
patagium in American English
1. a wing membrane, as of a bat. 2. the extensible fold of skin of certain insects or of a gliding mammal or reptile, as a flying squirrel.
Explanation:
A prehensile tail pertains to the tail of certain animals that enable the latter to grasp or hold objects. ... A fully prehensile tail is one in which the animal can fully hold on to and manipulate an object with its tail.
be able to explain the chemistry behind the edta titrations. why do we need the buffer? why do we spike the samples with mgedta? write the reactions to help explain.
Answers
EDTA (ethylenediaminetetraacetic acid) titrations involve the use of a buffer and spiking the samples with MgEDTA. The buffer is necessary to maintain a constant pH throughout the titration, as the reaction between EDTA and metal ions is pH-dependent. Spiking the samples with MgEDTA is done to provide a known concentration of EDTA and ensure that all metal ions in the sample are complexed before the titration. This allows for accurate determination of the metal ion concentration.
In EDTA titrations, EDTA forms stable complexes with metal ions through coordination bonds. The reaction between EDTA and metal ions is pH-dependent, and to maintain a constant pH, a buffer is used. The buffer helps resist changes in pH and ensures that the reaction proceeds under controlled conditions. By spiking the samples with MgEDTA, a known concentration of EDTA is introduced. This ensures that all metal ions in the sample are complexed with EDTA before the titration begins. The metal-EDTA complexes formed are typically highly stable, allowing for accurate determination of metal ion concentration. The reaction between EDTA and metal ions can be represented by the following general equation:
M^2+ + EDTA^4- → M-EDTA^2-
In this equation, M represents the metal ion, and EDTA^4- represents the fully deprotonated form of EDTA.
To learn more about EDTA (ethylenediaminetetraacetic acid) : brainly.com/question/13422325
#SPJ11
based on electronegativity differences, predict the identity of the polyatomic anion.
Answers
To predict the identity of a polyatomic anion based on electronegativity differences, we need to consider the relative electronegativities of the atoms that make up the anion.
An electronegative atom has a strong attraction for electrons, and in a chemical bond, it will attract electrons towards itself. The greater the electronegativity difference between two atoms, the more polar the bond between them is.
In the case of polyatomic anions, we need to look at the central atom and the surrounding atoms. The central atom usually has a higher electronegativity than the surrounding atoms. For example, in the sulfate ion (SO4 2-), the central sulfur atom has a higher electronegativity than the surrounding oxygen atoms. This creates a polar bond between the sulfur and oxygen atoms, resulting in a negatively charged ion.
Therefore, to predict the identity of a polyatomic anion, we need to look at the central atom and determine which atom has a higher electronegativity. This will help us predict the overall charge and identity of the anion.
Learn more about electronegativity here:
https://brainly.com/question/3393418
#SPJ11
undiluted sidestream smoke contains higher concentrations of toxic compounds than mainstream smoke. true false
Answers
Yes, the statement is true, undiluted side-stream smoke contains higher concentrations of toxic compounds than mainstream smoke.
The fatal potential of substances like lead, formaldehyde, asbestos, and arsenic is widely known. However, understand the specific health effects of exposure or the threshold at which they become toxic. What about the toxic substances we learned about in the news, like mercury in canned tuna, BPA in plastic, and cadmium in jewelry. The Toxic Compounds Portal offers a search engine that enables users to find chemicals by name, CAS number (Chemical Abstract Service registration number), synonym, or trade name. Users have access to additional substance search techniques. Substances that could have an adverse effect on organ systems' health (e.g., cardiovascular or respiratory systems) The chemical class includes metals, pesticides, and volatile organic substances. Individuals in the area looking for precise information on pollutants and how they affect health professionals in toxicology and public health looking for information on toxicity and associated health risk level experts who respond to cases of severe chemical exposure, such as emergency room physicians.
To know more about toxic please refer: https://brainly.com/question/28459917
#SPJ4
NH4, C032. OH", and PO43- are all examples of
Answers
Answer:
the popultion of it
Explanation:
NH₄+ , (CO₃)₂- , OH- , (PO₄)₃- are all examples of polyatomic ions.
What are Polyatomic ions?A covalent bound type of collection of two or more atoms or of a metal complex that can be said as behaving as a single unit and that has a net charge which is not zero is referred to as a polyatomic ion or a molecular ion.
Depending on the terminology , a polyatomic ion may or may not be referred to as a molecule. The Greek word poly- that means "many," although even ions made of just two atoms are frequently referred to be polyatomic.
A polyatomic ion may also be referred to as a radical (or less commonly, as a radical group).
The term "radical" today refers to a variety of free radicals, which are species with an unpaired electron and do not require a charge.
The hydroxide ion, which has the chemical formula OH- and is made up of one oxygen and one hydrogen atom and has a net charge of 1, is a straightforward example of a polyatomic ion.
A nitrogen atom, four hydrogen atoms, and a charge of +1 make up an ammonium ion, which has the chemical formula NH₄+.
In the context of acid-base chemistry and the production of salts, polyatomic ions are frequently helpful.
A polyatomic ion is frequently thought of as the conjugate base or acid of a neutral molecule. For instance, the polyatomic hydrogen (H₂) sulphate anion (SO₄) is the conjugate base of sulfuric acid (H₂SO₄). The sulphate anion (SO₄)₂ is created when another hydrogen ion is eliminated.
To know more about the similar questions on 'polyatomic ion'
visit- https://brainly.com/question/16843545
#SPJ2
In an isotope, what does the 'u' stand for?
For example: Lithium isotope: Li-6 6u
The u after 6
Answers
It is a unit of weight
Like we have kilograms,grams,milligrams, On the atomic level,the unit of weight measurement is AMU or just u for short.
For biomass, solar, coal, natural gas, oil, and geothermal energy, identify each energy resource as renewable or non-renewable.
Answers
Answer:
There are nine major kinds of natural resources found on the planet Earth, and all these nine major resources come under two categories, that is, renewable and nonrenewable. The renewable resources refer to the resources that get regenerated again and again over a short time duration. These include five major resources, that is, wind, solar, hydro, geothermal, and biomass.
On the other hand, nonrenewable energy resources are the ones that are present in a very limited amount, as it takes a very long time to regenerate again. The general kinds of nonrenewable energy resources are nuclear, coal, oil, and natural gas.
Hence, biomass, solar, and geothermal energy comes under the renewable resources category, and coal, natural gas, and oil come under renewable resources category.
Answer: the verified answer from an expert.
Explanation:
If distance, d, is proportional to time, t, and at time t =2.00 seconds, the distance is 6.20 m, find the distance when t = 3.50 s.
Answers
Find 1/4th of 2.00 and add that to 6.2
What can you say about an atom with a -2 ion of oxygen-17? WILL GIVE BRAINLIEST
Answers
Answer:
The mass number of an isotope is indicated in the name of the isotope by writing the name of the element followed by a hyphen and the mass number.
Explanation:
Examples include carbon-14, uranium-235, and oxygen-17.
What do nuclear reactions change in the atom? covalent bonds ionic bonds electrons neutrons protons
Answers
A nuclear reaction changes the number of protons and or neutrons in an atom.
What do nuclear reactions change in the atom?A nuclear reaction is a type of reaction that results in the change of the nucleus of an atom. We know that a nucleus of an atom is made up of protons and neutrons.
So we can conclude that a nuclear reaction changes the number of protons and or neutrons in an atom.
Learn more about reaction here: https://brainly.com/question/26018275
#SPJ4
Answer: Nuclear reactions happen inside the nucleus,so it changes the protons and neutrons
Explanation:
Which of the following situations is unique to hurricanes and not tornadoes?
form over land
form over warm waters
destroy buildings
form circular wind patterns
It’s science
Answers
Answer:
i think b.
Explanation:
At 2500 K, Kp is equal to 20 for the reaction Cl2(g) + F2(g) ⇌ 2 CIF(g) An analysis of a reaction vessel at 2500 K reavealed the presence of 0.18 atm Cl2, 0.31 atm F2, and 0.92 atm CIF. What will tend to happen to CIF as the reaction pro- ceeds toward equilibrium?
Answers
CIF will tend to increase as the reaction proceeds toward equilibrium.
Given that Kp is equal to 20 at 2500 K, we can calculate the initial concentrations of CIF using the ideal gas law. Let's assume the initial volume is 1 liter for simplicity.
For Cl2:
P(Cl2) = 0.18 atm
n(Cl2) = P(Cl2) * V / (RT) = 0.18 mol
For F2:
P(F2) = 0.31 atm
n(F2) = P(F2) * V / (RT) = 0.31 mol
For CIF:
P(CIF) = 0.92 atm
n(CIF) = P(CIF) * V / (RT) = 0.92 mol
Based on the balanced equation, for every 1 mole of CIF, 1 mole of Cl2 and 1 mole of F2 are consumed. Therefore, the initial moles of CIF are equal to the initial moles of Cl2 and F2.
Since the initial concentrations of CIF, Cl2, and F2 are the same, and the reaction is not at equilibrium, we can conclude that CIF will tend to increase as the reaction proceeds toward equilibrium. This is because the reaction favors the formation of CIF, as indicated by the value of Kp. As CIF forms, the concentrations of Cl2 and F2 decrease, driving the reaction in the forward direction to restore equilibrium.
for more questions on CIF
https://brainly.com/question/28297792
#SPJ8
The heart is a large
________________
that pumps the blood
throughout the body.
Answers
What orbital does the quantum numbers n=4 l=2 ml=2 describe
Answers
Answer:
Orbital designation
for Principle Quantum number n=4, this is the shell number & corresponds to N shell, (shells are designated K,L,M,N)
for Angular Momentum number l=2, this is the orbital level corresponding to d, given that l can be any integer 0-(n-1), for( 0,1,2,3 )corresponds to (s, p, d, f)
The orbital designation is 4d
for Magnetic Quantum number ml, the suborbitals correspond to -l through l, (-2,-1,0, 1,2)
for ml=-1 the suborbital -1 in the 4d sub shell with electron spin state ms=-1/2
In the equation - 8Fe + S8 --> 8 FeS
How many Fe atoms are there in 8 FeS
Answers
Answer:
4.81 * 10^24
Explanation:
we know that:
no. of atoms = moles * avogadro number
no. of moles = 8 * 6.022* 10^23
no. of moles = 4.81 * 10^24
Assign oxidation numbers to each atom in KBrO4
Answers
Answer:
K: +1
Br: +7
O: -2
Explanation:
O is always -2
K is in group one, so that is its charge
-8+1 is -7, so we flip the sign and that becomes Br
The reason why we flip it is to get a net balance of 0
Plz plz here
A student pours 25.0 mL of water into a graduated cylinder. She drops a plece of unknown metal with a mass of 18.9 g Into the cylinder. The
water level rises to 32.0 mL. The student uses the table to identify the metal
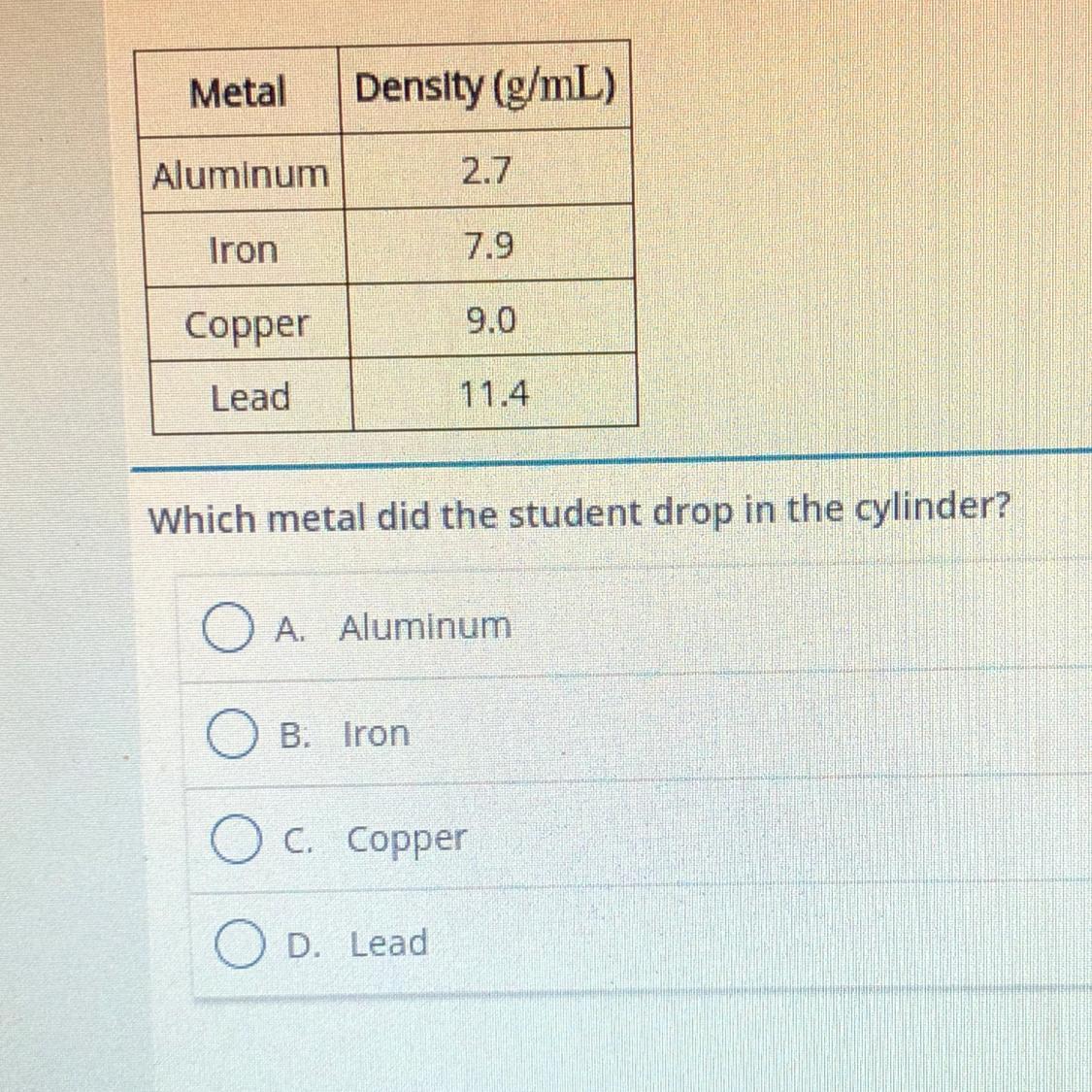
Answers
Answer:
Aluminum
Explanation
The volume of the metal is found by subtracting the final volume by the Intel volume giving 7
And the formula for density is mass divided by volume hence minus 7 by 18.9 giving 2.7
How does the octet rule apply to ionic and covalent bonds?
Select all that apply. A. Carbon with 4 valence electrons can satisfy the octet rule by gaining one electron from four different hydrogen atoms, each with 1 valence electron. B. Carbon with 4 valence electrons can satisfy the octet rule by sharing one electron with four different hydrogen atoms, each with 1 valence electron. C. Chlorine with 7 valence electrons can satisfy the octet rule by sharing one electron with a lithium atom with only 1 valence electron. D. Chlorine with 7 valence electrons can satisfy the octet rule by gaining one electron from a lithium atom with only 1 valence electron.
Answers
The octet rule is applicable to both ionic and covalent bonding. The appropriate responses to the above question are -
B. Carbon with 4 valence electrons can satisfy the octet rule by sharing one electron with four different hydrogen atoms, each with 1 valence electron.
D. Chlorine with 7 valence electrons can satisfy the octet rule by gaining one electron from a lithium atom with only 1 valence electron.
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a full outer shell of 8 electrons (or 2 electrons for hydrogen).
Option A is incorrect because it suggests that the carbon atom gains one electron from each of the four hydrogen atoms, which would result in the carbon atom having 8 electrons in its outer shell, violating the octet rule.
Option C is incorrect because it suggests that the chlorine atom shares one electron with the lithium atom, which would result in the chlorine atom having only 7 electrons in its outer shell, also violating the octet rule.
For such more questions on Covalent bonding.
https://brainly.com/question/12732708
#SPJ4
Why are ionic compounds not considered individual molecules?
Answers
Molecules are compounds that make covalent bonds between atom. In a covalent bond, the electrons from each atom invoved are shared between them, sticking the atoms together.
In ionic compounds, the electrons of each atoms are not shared, they are part of either of the ions. The ions sticke together because they are charged with opposite charges, so they attract each other.
So, ionic compounds are not considered individual molecules because they don't make covalent bonds, that is, their atoms don't shared electrons, they attract each other because of their opposite charges.
which atomic orbitals from carbon hybridize to form the bonds in ch₄?
Answers
In CH₄ (methane), the carbon atom undergoes sp³ hybridization to form four hybrid orbitals, each of which is used to form a single covalent bond with a hydrogen atom.The atomic orbitals from carbon that hybridize to form the bonds in CH₄ are one 2s and three 2p orbitals.
To obtain these sp³ hybrid orbitals, one 2s orbital and three 2p orbitals of carbon are hybridized. The hybridization process involves mixing the 2s and three 2p orbitals to form four new hybrid orbitals with equal energy, which are called sp³ orbitals.
The sp³ orbitals are arranged in a tetrahedral geometry around the carbon atom, with each orbital pointing towards one of the hydrogen atoms. The four hydrogen atoms then bond with the carbon atom through the overlap of their 1s atomic orbitals with the four sp³ hybrid orbitals of carbon.
So, the atomic orbitals from carbon that hybridize to form the bonds in CH₄ are one 2s and three 2p orbitals.
learn more about hybridization here:
https://brainly.com/question/14140731
#SPJ4
describe a type of chemical indicator used each time on packagin toi identify instruments that have been processed
Answers
All sterilizing methods, including steam, hydrogen peroxide, and ethylene oxide, should employ chemical indicators.
What is sterilizing ?Any procedure that eliminates, eradicates, or renders inactive any life forms—particularly microbes like fungi, bacteria, spores, and unicellular eukaryotic organisms—present in or on a given surface, object, or fluid is referred to as sterilization.
Despite the fact that there are several physical and chemical procedures, just a handful are essential for effectively sterilizing equipment. Having said that, there are currently three main categories of sterilizing procedures used in science. They are sterilizing using ethylene oxide, dry heat, and steam (EtO).
To learn more about sterilizing from the given link:
brainly.com/question/14290729
#SPJ4
at over 1700 miles thick the ___ contains superheated rocks and minerals
Answers
At over 1700 miles thick the lower mantle contains superheated rocks and minerals.
What is Mantle?The mantle is the mostly-solid bulk of Earth's interior. The mantle lies between Earth's dense, super-heated core and its thin outer layer, the crust.
As Earth began to take shape about 4.5 billion years ago, iron and nickel quickly separated from other rocks and minerals to form the core of the new planet. The molten material that surrounded the core was the early mantle.
The lower mantle extends from about 660 kilometers (410 miles) to about 2,700 kilometers (1,678 miles) beneath Earth’s surface. The lower mantle is hotter and denser than the upper mantle and transition zone.
Therefore, at over 1700 miles thick the lower mantle contains superheated rocks and minerals.
Learn more about Mantle, here:
https://brainly.com/question/28827790
#SPJ9
số oxi hóa của các nguyên tố: Fe(OH)2; Fe(OH)3; FexOy; Zn(OH)2 là gì
Answers
Answer:
esfsef
Explanation:
esfsefseF
