an unknown radioactive substance has a half-life of 3.20 hours . if 21.1 g of the substance is currently present, what mass a0 was present 8.00 hours ago?
Answers
Total, 119.31 grams of the radioactive substance were present 8.00 hours ago.
To determine the initial mass (\(a_{0}\)) of the radioactive substance 8.00 hours ago, we can use the formula for exponential decay;
a = \(a_{0}\) × \((1/2)^{(t/T)}\)
where; a = current mass
\(a_{0}\) = initial mass
t = elapsed time
T = half-life of the substance
Given;
a = 21.1 g (current mass)
t = 8.00 hours
T = 3.20 hours (half-life)
We need to solve for \(a_{0}\), the initial mass.
Rearranging the formula;
\(a_{0}\) = a × \(2^{(t/T)}\)
Substituting the given values;
a0 = 21.1 g × \(2^{(8.00 hours/3.20 hours)}\)
Calculating the exponent;
8.00 hours / 3.20 hours = 2.5
Now we can calculate \(a_{0}\);
\(a_{0}\) = 21.1 g × \(2^{(2.5)}\)
\(a_{0}\) = 21.1 g × 5.6568542
\(a_{0}\) ≈ 119.31 g
Therefore, approximately 119.31 grams of mass are present.
To know more about radioactive substance here
https://brainly.com/question/31636631
#SPJ4
Related Questions
why is it important to not dispose of medicine this way
Answers
Answer:
Because Environmental Risks
Explanation:
Medicines poured down the drain can enter the environment and your community’s drinking water supplies. And it can cause a lot of diseases!
What is the process of transforming raw materials collected by extractive companies into products called?
Answers
The process of turning the raw materials gathered by mining corporations into finished goods is known as material processing.
What does raw material processing entail?Manufacturing is the process of converting raw materials or component components into completed products using tools, labor, machinery, and chemical processing.
Why is the processing of materials important?The objective of material processing is to create the structural characteristics (such as crystal structure, microstructure, size, and shape) required for the product to function effectively in its intended use. Processing materials is essential to production and is at the heart of the area of materials science and engineering.
Why are materials processed?We manipulate materials to improve their strength, durability, water proofness, or even merely their aesthetic appeal. When new materials are created by combining other components, they are sometimes referred to as mixtures.
learn more about material processing here
https://brainly.com/question/555478
#SPJ4
A 0.100 m solution of which one of the following solutes will have the lowest vapor pressure?
a. sucrose
b. Al(ClO4)3
c. NaCl
d. KClO4
e. Ca(ClO4)2
Answers
To determine which 0.100 m solution will have the lowest vapor pressure, we need to consider the number of solute particles each substance will produce when dissolved in water. The more solute particles present, the lower the vapor pressure.
a. Sucrose: Since it is a non-electrolyte, it will not dissociate in water. Number of particles produced = 1.
b. Al(ClO₄)₃: When dissolved, it will dissociate into 1 Al³⁺ ion and 3 ClO₄⁻ ions. Number of particles produced = 1 + 3 = 4.
c. NaCl: When dissolved, it will dissociate into 1 Na⁺ ion and 1 Cl⁻ ion. Number of particles produced = 1 + 1 = 2.
d. KClO₄: When dissolved, it will dissociate into 1 K⁺ ion and 1 ClO₄⁻ ion. Number of particles produced = 1 + 1 = 2.
e. Ca(ClO₄)₂: When dissolved, it will dissociate into 1 Ca²⁺ ion and 2 ClO₄⁻ ions. Number of particles produced = 1 + 2 = 3.
The 0.100 m solution of Al(ClO₄)₃ (option b) will have the lowest vapor pressure, as it produces the highest number of solute particles (4) when dissolved in water.
To know more about Solute :
https://brainly.com/question/13812915
#SPJ11
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
A gas is heated from 213.0 K to 398.0 K and the volume is increased from 13.0 liters to 35.0 liters by moving a large piston within a cylinder. If the original pressure was 3.15 atm, what would the final pressure be?
Answers
Answer:
P₂ = 0.13880 atm
Explanation:
Given info are;
Initial volume = 13.0 L
Initial pressure = 3.15 atm
Initial temperature = 213.0 K
Final temperature = 398.0 K
Final volume = 35.0 L
Final pressure = ?
Note;
Base on general gas equation:
P₁V₁/T₁ = P₂V₂/T₂
Formula = P₁V₁/T₁ = P₂V₂/T₂
P₁ = Initial pressureV₁ = Initial volumeT₁ = Initial temperatureP₂ = Final pressureV₂ = Final volumeT₂ = Final temperatureSolution:
P₂ = P₁V₁ T₂/ T₁ V₂
P₂ = 3.15 atm x 13.0 L x 398.0k/213.0 k x 35.0 L
P₂ = 1034.8 atm .L.K/ 7455 K.L
P₂ = 0.13880617035
P₂ = 0.13880 atm
When we multiply or divide the values the number of significant figures must be equal to the less number of significant figures in given value.
Thus we will include five significant figures in answer because 1034.8 have five significant figure.
[RevyBreeze]
Ideal gas law is valid only for ideal gas not for vanderwaal gas. Combined Boyle's and Charles' gas law is used here. Therefore the new pressure of gas is 0.13880 atm .
What is ideal gas equation?Ideal gas equation is the mathematical expression that relates pressure volume and temperature.
Mathematically the relation between Pressure, volume and temperature can be given as
PV=nRT
where,
P = pressure of gas
V= volume of gas
n =number of moles of gas
T =temperature of gas
R = Gas constant = 0.0821 L.atm/K.mol
Combining Boyle's and Charles' gas law
P₁V₁/T₁ = P₂V₂/T₂
P₂ = P₁V₁ T₂/ T₁ V₂
P₂ = 3.15 atm x 13.0 L x 398.0k/213.0 k x 35.0 L
P₂ = 1034.8 atm .L.K/ 7455 K.L
P₂ = 0.13880617035
P₂ = 0.13880 atm
Therefore the new pressure of gas is 0.13880 atm when the temperature is changed to 398.0 K and volume is increased to 35.0 liters.
To learn more about ideal gas equation, here:
https://brainly.com/question/14826347
#SPJ2
name two noble gasses
Answers
Answer:
The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og).
Explanation:
How many moles of N2 in 57.1 g of N2?
Answers
We are given –
Mass of \( \bf N_2\) is 57.1 g and we are asked to find number of moles present in 57.1 g of \( \bf N_2\)\(\qquad\)\( \pink{\bf\longrightarrow { Molar \:mass \:of \: N_2:-} }\)
\(\qquad\)\( \bf \twoheadrightarrow 14\times 2 \)
\(\qquad\)\( \bf \twoheadrightarrow 28\)
\(\qquad\)____________________
Now,Let's calculate the number of moles present in 57.1 g of \( \bf N_2\)
\(\qquad\)\( \purple{\bf\longrightarrow { No \:of \:moles = \dfrac{Given \:mass}{Molar\: mass}}}\)
\(\qquad\)\( \bf \twoheadrightarrow \dfrac{57.1}{28}\)
\(\qquad\)\( \bf \twoheadrightarrow 2.04\: moles\)
__________________________________
increasing the airplane's speed or wing size does which of the following
a) decreases the amount of drag on the plane
b) increases the gravitational pull on the pane
c) generates more lifting force
d) creates a sonic boom
Answers
Answer:
increases the gravitional pull on tge pane
Answer:
generates more lifting on the plane
Explanation:
Cracking of long saturated hydrocarbon chain molecule C40H82 produces 3 octane molecules and the rest as ethane molecules. How many moles of hydrogen are needed to crack one mole of this long hydrocarbon chain? Give your answer in whole numbers.
Answers
To determine the number of moles of hydrogen needed to crack one mole of the long saturated hydrocarbon chain (C40H82), we can analyze the reactants and products involved in the cracking reaction.
The cracking reaction is given as: C40H82 -> 3 C8H18 + n C2H6. From the equation, we can see that one mole of the long hydrocarbon chain (C40H82) produces three moles of octane (C8H18) and n moles of ethane (C2H6). Since the cracking process involves breaking the carbon-carbon bonds and forming new carbon-hydrogen bonds, the number of hydrogen atoms in the products should remain the same as in the reactant.
The long hydrocarbon chain (C40H82) contains 82 hydrogen atoms, and the products, 3 moles of octane (C8H18), contain (3 moles) * (18 hydrogen atoms/mole) = 54 hydrogen atoms. Therefore, the number of moles of hydrogen needed for cracking one mole of the long hydrocarbon chain can be calculated as: Number of moles of hydrogen = 82 - 54 = 28 moles. Hence, 28 moles of hydrogen are required to crack one mole of the long saturated hydrocarbon chain (C40H82).
To learn more about number of moles click here: brainly.com/question/20370047
#SPJ11
C₆H₁₂O₆ + 6O₂→6CO₂ + 6H₂O + Energy
Answers
Answer:
C₆H₁₂O₆ + 6O₂→6CO₂ + 6H₂O + Energy=chemical equation for cellular respiration
Explanation:
hope this help
this is james not jade
Compare ferns, gymnosperms, and angiosperms by writing each characteristic in the box underneath the correct plant type. • reproduce with cones • reproduce with spores • reproduce with flowers • existed the longest in Earth history • newest type of plant in Earth history • has needle-like, waxy leaves 1)

Answers
Ferns reproduce with spores which existed the longest in Earth history.
Gymnosperms reproduce with cones and has needle-like, waxy leaves.
Angiosperms reproduce with flowers and newest type of plant in Earth history.
How ferns, gymnosperm and angiosperm reproduce?Ferns reproduce with spores that existed the longest in Earth, gymnosperms reproduce through the formation of cones having needle-like, waxy leaves whereas angiosperms reproduce with flowers which turns into seed. The newest type of plant in Earth history is also belongs to this group.
Learn more about spore here: https://brainly.com/question/1022692
Which element does the "X" in
the isotope notation
represent?
14X
6
A. C
B. O
C. Si
D. N
Answers
The element whose isotope is represented as \(_{6}^{14}X\) is carbon -14. The atomic number of carbon is 6 and the mass number for the given isotope is 14 amu.
What are isotopes?Isotopes are atoms of same element with different mass numbers. Isotopes slightly changes in chemical and physical properties between each other.
Almost elements in the periodic table are having isotopes. However not all of them are stable. Some of them for heavy metals are radioactive isotopes and are not stable.
The isotope is represented by showing mass number in top of the symbol and atomic number at the bottom. The atomic number 6 corresponds to carbon. Hence, option A is correct. Carbon -14 isotope is given here.
To find more on isotopes, refer here:
https://brainly.com/question/27475737
#SPJ2
Calculate the number of atoms in 6.5 moles of NaCI
Answers
Answer:
\(1 \: mole = 6.02 \times {10}^{23} \: atoms \\ 6.5 \: moles = (6.5 \times {6.02 \times 10}^{23} ) \: atoms \\ = 3.913 \times {10}^{24} \: atoms\)
If anyone have the answers to this plz tell me

Answers
Explanation:
hey can I please get the passage so that I can help you
: Axit sunfurơ (H2SO3) là axit yếu, ngay trong dung dịch H2SO3 cũng bị phân hủy thành chất X và H2O. X là:
A. H2S B. SO2 C. H2SO4 D. SO3
Answers
Answer:
DD
Explanation:
how many liters are in 1 mole of oxygen gas?
Answers
Answer:
22.4L
Explanation:
Glad to help :)
Magnesium oxide (mgo) forms when the metal magnesium burns in air.(a) if 1.18 g of mgo contains 0.712 g of mg, what is the mass ratio of magnesiu?
Answers
The mass ratio of magnesium in magnesium oxide (MgO) can be calculated by dividing the mass of magnesium (0.712 g) by the mass of magnesium oxide (1.18 g).
To find the mass ratio, we divide the mass of the element of interest (magnesium) by the mass of the compound (magnesium oxide). In this case, the mass of magnesium is given as 0.712 g and the mass of magnesium oxide is given as 1.18 g. So, the mass ratio of magnesium is calculated as follows:
Mass ratio = mass of magnesium / mass of magnesium oxide
= 0.712 g / 1.18 g
Calculating this gives us the mass ratio of 0.604.
Therefore, the mass ratio of magnesium in magnesium oxide is approximately 0.604.
The mass ratio of magnesium in magnesium oxide can be found by dividing the mass of magnesium by the mass of magnesium oxide. In this case, the mass of magnesium is given as 0.712 g and the mass of magnesium oxide is given as 1.18 g. By dividing these two values, we get a mass ratio of approximately 0.604. This means that for every gram of magnesium oxide, there are approximately 0.604 grams of magnesium. This mass ratio is useful in determining the composition of compounds and can be used in various chemical calculations.
To know more about magnesium visit:
https://brainly.com/question/8351050
#SPJ11
FILL IN THE BLANK.Of the molecules below, the bond in ____ is the most polar. A) HBr B) HI C) HCl D) HF E) H2
Answers
Of the molecules given below, the bond in HF is the most polar. Option D) is correct.
Polarity is the extent to which different atoms' electrons are shared in a chemical bond. In a molecule, the unequal distribution of charge leads to a dipole moment. The greater the electronegativity difference between the bonded atoms, the more polar the bond and molecule become.
A polar bond is created when two atoms with different electronegativity values join together. The atom with a greater electronegativity has a stronger pull on the shared electrons in the bond, resulting in a partial negative charge, while the atom with a lower electronegativity has a partial positive charge.
Fluorine is the most electronegative element on the periodic table. The electronegativity values of the elements in the bond between HF are 2.20 and 0.98 for fluorine and hydrogen, respectively. Because there is such a significant difference in electronegativity, the bond between them is highly polar.
Hence, of the molecules listed above, the bond in HF is the most polar. Hence, option D) is correct.
To know more about molecules refer here :
https://brainly.com/question/1078183
#SPJ11
When carbon disulfide, CS2, forms from its elements. Heat is absorbed. How much heat would be required to produce 5.0 moles of carbon disulfide
Answers
Answer:
5.9 × 10² kJ
Explanation:
When carbon disulfide, CS₂, forms from its elements, heat is absorbed. The corresponding value for the standard enthalpy of formation of carbon disulfide is 117.36 kJ/mol. The thermochemical equation that represents this process is:
C(graphite) + 2 S(s, rhombic) ⇒ CS₂(g) ΔH°f = 117.36 kJ/mol
117.36 kJ of heat are absorbed when 1 mole of CS₂ is formed. The amount of heat absorbed when 5.0 moles of CS₂ are formed is:
5.0 mol × 117.36 kJ/mol = 5.9 × 10² kJ
9. t An element that has only 5 electrons in the 4p orbital is located in which group? A. O Group 1 B. Group 17 C. O Group 2 D. Group 8
Answers
Answer:
B. Group 17
Explanation:
The element is Bromine. Its electron configuration is:
1s2 2s2p6 3s2p6d10 4s2p5
You can see that its last orbital is 4p^5 for 5 electrons in the 4p orbital.

n-(2-hydroxy-3-methoxybenzyl)-n-(p-tolyl)acetamide
Answers
The systematic name of this molecule is 4-((2-hydroxy-3-methoxybenzyl)amino)-N-(p-tolyl)benzamide.
1. First, the molecule should be broken down into its component parts: the amide group, the amino group, the benzene ring, the tolyl group, and the hydroxy-methoxybenzyl group.
2. The amide group is composed of an acyl group (the N-(p-tolyl)acetamide part) and an amine group (the N-(2-hydroxy-3-methoxybenzyl) part).
3. The acyl group is composed of an acyl chain (the p-tolyl group) and an acid group (the acetamide group).
4. The amine group is composed of an amino group (the 2-hydroxy-3-methoxybenzyl group) and an alkyl group (the N part).
5. The benzene ring is composed of a phenyl group (the benzene ring) and a substituent (the tolyl group).
6. Using this information, the systematic name of the molecule can be constructed. The systematic name is 4-((2-hydroxy-3-methoxybenzyl
learn more about molecule here
https://brainly.com/question/19556990
#SPJ4
A covalent compound contains 2 atoms of nitrogen and 4 atoms of oxygen. What is the percent by mass
composition of nitrogen?
(I’ll mark you as brainlister)
Answers
The percent by mass composition of nitrogen:
Given information :Nitrogen- 2 atomsOxygen-4 atomsMass of oxygen-16Mass of nitrogen -14Solution :
Total mass = Nitrogen + Oxygen
Total mass= 2*14+4*16Total mass= 92Percent mass composition of nitrogen =mass of nitrogen*2/ total mass*100
Percent mass composition of nitrogen=28/92*100Percent mass composition of nitrogen=30*.4The percent by mass composition of nitrogen is 30.4.
Learn more :
https://brainly.com/question/4412816?referrer=searchResults
what is your definition of science?
**ANSWER AND ILL GIVE YOU BRAINLIEST ANSWER**
Answers
Balancing Equations. How many of the element should I drag to the center?
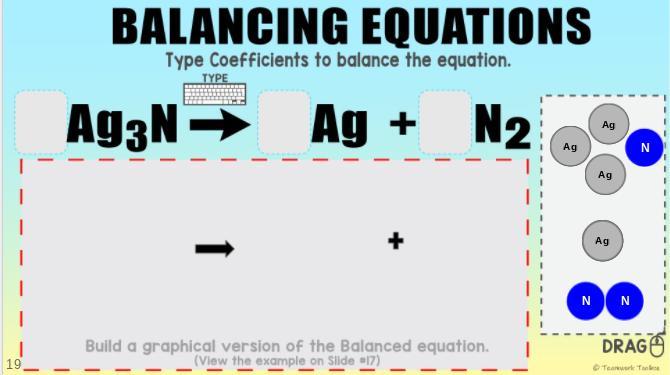
Answers
Answer:
2, 6, 1
Explanation:
Ag3N is dissolved into Ag and N2. Since N2 is a molecule, there should be two Ag3Ns.
Nickel has a cubic unit cell. The edge of the unit cell is 3.524
x 10^(-8)cm. Determine the atomic radius of Nickel.
Answers
The approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
In a cubic unit cell, the body diagonal length (diagonal that passes through the center of the unit cell) is equal to four times the atomic radius (4r). We can use this relationship to find the atomic radius of nickel.
Given: Edge length of the unit cell (a) = 3.524 × 10^(-8) cm
The body diagonal length is given by:
Diagonal length (d) = a√3
Substituting the given values:
d = (3.524 × 10^(-8) cm) × √3
Now, we can calculate the atomic radius (r) by dividing the diagonal length by 4:
r = d / 4
Performing the calculations:
r = [(3.524 × 10^(-8) cm) × √3] / 4
r ≈ (3.524 × 10^(-8) cm) × (1.732 / 4)
r ≈ 1.532 × 10^(-8) cm
Therefore, the approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
Know more about atomic radius here
https://brainly.com/question/18095927#
#SPJ11
your lab write-up, three possibilities for the mechanism of the rate determining step were listed. 1. The rate-determining step has two iodide ions coming together. 2. The rate-determining step involves a persulfate ion decomposing. 3. The rate-determining step has an iodide ion and a persulfate ion coming together. Which mechanism did your experiment confirm? the third . (a) If the first mechanism is correct, what should happen to the rate if the concentration of iodide ion is doubled and other concentrations are held constant? (b) If the first mechanism is correct, what should happen to the rate if the concentration of persulfate ion is doubled and other concentrations are held constant? (c) If the second mechanism is correct, what should happen to the rate if the concentration of iodide ion is doubled and other concentrations are held constant? (d) If the second mechanism is correct, what should happen to the rate if the concentration of persulfate ion is doubled and other concentrations are held constant?
Answers
Your lab write-up confirmed that the third mechanism, which involves an iodide ion and a persulfate ion coming together, is the rate determining step for your experiment.
If the first mechanism is correct (two iodide ions coming together), then doubling the concentration of iodide ions would increase the rate of the reaction. This is because the rate-determining step involves two iodide ions coming together, so increasing the concentration of iodide ions would increase the likelihood of this step occurring.
If the first mechanism is correct, then doubling the concentration of persulfate ions would not have any effect on the rate of the reaction. This is because the rate-determining step does not involve persulfate ions, so increasing their concentration would not affect the rate.
If the second mechanism is correct (persulfate ion decomposing), then doubling the concentration of iodide ions would not have any effect on the rate of the reaction. This is because the rate-determining step does not involve iodide ions, so increasing their concentration would not affect the rate.
If the second mechanism is correct, then doubling the concentration of persulfate ions would increase the rate of the reaction. This is because the rate-determining step involves persulfate ions decomposing, so increasing their concentration would increase the likelihood of this step occurring.
To know more about iodide ions, refer
https://brainly.com/question/31265404
#SPJ11
How many grams of nh3 are needed to provide the same number of molecules as in 0. 65 g of sf6 ?.
Answers
Grams of nh3 are needed to provide the same number of molecules as in 0. 65 g of sf6 is 0.09 grams.
A mole is a very important unit of measurement that chemists use. A mole of something way you have got 602,214,076,000,000,000,000,000 of that thing, like how having a dozen eggs method you've got twelve eggs. Chemists have to degree the use of moles for very small such things as atoms, molecules, or different debris.
calculation:-
Avogadro's number A = 6.022 x 1023
Molecules of NH3 = Molecules of SF6
An x Weight of NH3 / Molecular Weight of NH3
= A x Weight of SF6 / Molecular Weight of SF6
Weight of NH3 = (Weight of SF6 / Molecular Weight of SF6) x Molecular Weight of NH3
= (0.75 / 146.05) x 17.03 = 0.09 grams
The weight of NH3 needed is 0.09 grams
The mole, image mol, is the unit of amount of substance within the international system of devices. the quantity of the substance is a measure of what number of elementary entities of a given substance in an item or sample. The mole is defined as containing exactly 6.02214076×10²³ basic entities
Learn more about moles here:-https://brainly.com/question/15356425
#SPJ4
HELPPP PLZZZZ :( ONLY ANSWER IF U ACUALLY KNOOOW ALL




Answers
Answer:
Net: 1N
Explanation:
1.) Net:1N, direction: left
2.) Net: 1N, direction: down
3.) Net: 0N, no motion
4.) Net: 1N, direction: up
5.) Net: 1N, direction: up
in regard to the e2 mechanism, rank the leaving groups in order of increasing reaction rate.
Answers
The rank of groups in order of increasing reaction rate in regards to E2 mechanism is F, Cl, Br and I.
Iodine is often non-reactive, while fluorine has an explosive reaction that makes it challenging to manage. Bromination and chlorination are typically exothermic reactions.
Group 7's halogens, which are non-metal elements, become less reactive as you move down the group. In contrast to the alkali metals in Group 1 of the periodic table, this trend is the opposite.
Halogens react at a rate of "F (2) gt Cl (2) gt Br (2) gt l (2)." Alkane react quickly with F2, but slowly with I2, necessitating the use of a catalyst. Fluorine's high electronegativity is the reason.
For more information on reactivity of halogens kindly visit to
https://brainly.com/question/24751735
#SPJ4
Complete question: in regards to the E2 mechanism rank the leaving groups of increasing reaction rate
a) F b)I c)Br d)Cl
A) What volume of a 0.268 M perchloric acid solution is required to neutralize 12.9 mL of a 0.128 M calcium hydroxide solution?
B) What volume of a 0.182 M potassium hydroxide solution is required to neutralize 25.4 mL of a 0.228 M perchloric acid solution?
Answers
A) 6.19 mL of 0.268 M perchloric acid solution is required to neutralize 12.9 mL of 0.128 M calcium hydroxide solution.
B) 31.7 mL of 0.182 M potassium hydroxide solution is required to neutralize 25.4 mL of 0.228 M perchloric acid solution.
A) To solve this problem, we need to use the balanced chemical equation for the reaction between perchloric acid and calcium hydroxide, which is:
HClO₄ + Ca(OH)₂ → Ca(ClO4)₂ + 2H₂O
From the equation, we can see that one mole of perchloric acid reacts with one mole of calcium hydroxide. Therefore, we can use the following formula to calculate the volume of perchloric acid solution required:
Molarity of perchloric acid x Volume of perchloric acid solution = Molarity of calcium hydroxide x Volume of calcium hydroxide solution
Plugging in the values given in the problem, we get:
0.268 M x Volume of perchloric acid solution = 0.128 M x 12.9 mL
Solving for Volume of perchloric acid solution, we get:
Volume of perchloric acid solution = (0.128 M x 12.9 mL) / 0.268 M = 6.19 mL
B) Similar to part A, we need to use the balanced chemical equation for the reaction between potassium hydroxide and perchloric acid, which is:
KOH + HClO₄ → KClO₄ + H₂O
From the equation, we can see that one mole of potassium hydroxide reacts with one mole of perchloric acid. Therefore, we can use the following formula to calculate the volume of potassium hydroxide solution required:
Molarity of potassium hydroxide x Volume of potassium hydroxide solution = Molarity of perchloric acid x Volume of perchloric acid solution
Plugging in the values given in the problem, we get:
0.182 M x Volume of potassium hydroxide solution = 0.228 M x 25.4 mL
Solving for Volume of potassium hydroxide solution, we get:
Volume of potassium hydroxide solution = (0.228 M x 25.4 mL) / 0.182 M = 31.7 mL
Learn more about volume: https://brainly.com/question/1578538
#SPJ11