An atom has the electron configuration of 2-8-2. An ion of the same element has electron configuration of 2-8. What would be the net charge of the ion?
1. -2
2.+12
3.+2
4.+10
Answers
Answer:
3. +2
Explanation:
Changing from 2-8-2 to 2-8 means it has lost 2 electrons. Since each electron is a negative 1, the net charge on the remaining ion would be +2.
Related Questions
Which Inorganic Substance has a melting point of -101°C and a boiling point of -35°C?
Hydrogen
Chlorine
Hydrogen Sulfide
Hydrogen Chloride
Answers
Hydrogen Chloride (HCl) is the inorganic substance that has a melting point of -101°C and a boiling point of -35°C.
Hydrogen Chloride is a colorless and pungent-smelling gas that is highly soluble in water and forms a strong acid when dissolved in water. It is commonly used in a variety of industrial applications, including the production of fertilizers, dyes, and plastics. Its melting and boiling points are relatively low, which allows it to exist as a gas at room temperature, making it easier to handle and transport. However, its corrosiveness and reactivity with many materials means that it must be handled with care and proper safety precautions must be taken when using it.
Learn more about Hydrogen Chloride here:
https://brainly.com/question/19053776
#SPJ4
CAN SOMEONE PLEASE ANSWER THIS FAST PLEASE!
How many moles of ammonia (NH3) can be produced from the reaction of 4.0 liters of hydrogen at 50.0°C and 1.2atm of pressure with excess nitrogen?
Answers
Answer: Therefore, approximately 0.1247 moles of ammonia can be produced from the given reaction.
Explanation:
To determine the number of moles of ammonia (NH3) produced from the given reaction, we need to use the ideal gas law and stoichiometry.
The balanced chemical equation for the reaction between hydrogen (H2) and nitrogen (N2) to form ammonia (NH3) is:
N2 + 3H2 → 2NH3
From the equation, we can see that three moles of hydrogen react with one mole of nitrogen to produce two moles of ammonia.
First, let's convert the given conditions of hydrogen to the appropriate units for the ideal gas law:
Volume of hydrogen = 4.0 liters
Temperature of hydrogen = 50.0°C = 50.0 + 273.15 = 323.15 K
Pressure of hydrogen = 1.2 atm
Now, let's calculate the number of moles of hydrogen using the ideal gas law equation:
PV = nRT
where:
P = pressure (in atm)
V = volume (in liters)
n = number of moles
R = gas constant (0.0821 L·atm/(mol·K))
T = temperature (in Kelvin)
n(H2) = PV / RT
n(H2) = (1.2 atm) * (4.0 L) / (0.0821 L·atm/(mol·K) * 323.15 K)
≈ 0.187 mol
Since the stoichiometry ratio is 3:2 (H2:NH3), we can conclude that 0.187 moles of hydrogen can produce (0.187/3) * 2 = 0.1247 moles of ammonia.
what is the result of applying too much sample to the tlc plate?
Answers
Answer:
If the TLC plate runs samples which are too concentrated, the spots will be streaked and/or run together. If this happens, you will have to start over with a more dilute sample to spot and run on a TLC plate.
Explanation:
consider a insoluble salt in which the absolute value of the heat of hydration is more than the absolute value of the lattice enthalpy. what are the signs of standard gibbs energy, enthalpy and entropy of precipitation? select the words positive, zero, negative, or unknown in each of the boxes when adding a solid salt to water.
Answers
When an insoluble salt is added to water, if the absolute value of the heat of hydration is greater than the absolute value of the lattice enthalpy, the standard Gibbs energy, enthalpy, and entropy of precipitation will all be negative.
Let's understand this in detail:
1. Lattice energy is the energy required to separate one mole of a crystalline solid into its constituent ions in the gas phase. Separating one mole of a crystalline solid into its constituent ions in the gas phase is known as sublimation or vaporization.
2. The formula for the Lattice Energy of an Ionic Compound can be given as:
Lattice Energy (U) = - k(q1q2)/d, where q1 and q2 are the charges of the ions, d is the distance between the centers of the two ions, and k is a constant with a value of 8.99 x 10^9 Jm/C^2.
3. Heat of hydration is the heat required for a mole of gaseous ions to be dissolved in sufficient water so that the solution is infinite dilution. The hydration of gaseous ions releases heat, which is known as the heat of hydration.
4. Enthalpy changes define exothermic and endothermic processes. When a chemical reaction releases energy in heat, it is exothermic. When a chemical reaction absorbs energy in the form of heat, it is endothermic. The standard Gibbs energy, enthalpy, and entropy of precipitation will all be negative when adding a solid salt to water if the heat of hydration is greater than the absolute value of the lattice enthalpy.
Learn more about exothermic reactions: Which answer defines exothermic reaction? https://brainly.com/question/2924714
#SPJ11
when you hold a metal coat hanger in a camp fire to roast a marshmallow, the coat hanger might get too hot to hold. what type of heat transfer is occurring between the metal coat hanger and your hand? explain what is happening at the molecular level that causes your hand to feel hot.
Answers
Answer:
Heat transfer by conduction
A neutral atom has a mass number of 119 and has 69 neutrons. write its isotopic symbol.
Answers
The Isotopic symbol of the element that has a mass number of 119 and has 69 neutrons is ₅₀¹¹⁹Sn.
What is atomic number and mass number?Atomic number is the number of protons present in the nucleus of the atom of an element.
The mass number is the sum of the protons and neutrons present in the nucleus of the atom of the element.
The mass number of the element = 119
The neutron number = 69
The atomic number = 119 - 69
Atomic number = 50
The element with atomic number 50 is Tin with symbol Sn.
The Isotopic symbol of the element is ₅₀¹¹⁹Sn.
Learn more about isotopic symbol at: https://brainly.com/question/160068
#SPJ1
How many different dichloroethene (C2H2F2) are there that differ in the location of their fluorine atoms?
Answers
There are two different dichloroethene (C2H2F2) molecules that differ in the location of their fluorine atoms.
The two isomers, known as cis and trans, have different molecular and physical properties due to their distinct molecular arrangements. In the cis isomer, both fluorine atoms are on the same side of the molecule, while in the trans isomer, the fluorine atoms are on opposite sides.
This difference in arrangement gives the two isomers different chemical and physical properties, such as boiling points, melting points, and reactivity towards other substances. These differences can impact their uses and applications, such as in refrigeration and as solvents.
Understanding the different isomers and their properties is important for making informed decisions in the production and use of chemicals, as well as for understanding their impact on the environment.
To know more about dichloroethene click here:
https://brainly.com/question/28296044#
#SPJ11
substance with 169 melting point
Answers
Answer:
the substance with 169 melting point is buten
suppose the sample of magnesium used in this lab was contaminated with another metal that does not react with hydrochloric acid. how would this have changed your results?
Answers
If the sample of magnesium used in a lab was contaminated with another metal that doesn't react with hydrochloric acid, then the results obtained in the experiment would be affected.
This is because the data collected during the experiment would reflect the reaction between hydrochloric acid and the contaminated sample instead of pure magnesium. As a result, the following changes in results might have been observed:
1. The mass of the contaminated sample would be higher than the mass of pure magnesium.
2. The rate of reaction between the contaminated sample and hydrochloric acid would be slower than the reaction between pure magnesium and hydrochloric acid.
3. The volume of hydrogen gas collected from the reaction would be lower than the volume of hydrogen gas collected in the reaction between pure magnesium and hydrochloric acid.
learn more about contaminated here
https://brainly.com/question/465199
#SPJ11
Define mixtures and compounds, and explain how, without tasting it, Nicole can test to make sure she is using the sugar-water in the glaze? PLSS ANSWER GUYSS
Answers
Answer:Is sugar water a mixture or a compound? Define mixtures and compounds, and explain how without tasting it, Nicole can test to make sure she is using the sugar water in the glaze. The sugar-water is a mixture because it is chemically bonded, not chemically combined. A mixture is something that has 2 substances or more. hope this helps
Explanation:
How did the Heisenberg uncertainty principle contribute to the idea that electrons occupy “clouds," or "orbitals”?
Answers
Answer:
Because the exact position of the electron is not known,it must be assumed that the electron takes up the entire space in an orbital.
Explanation:
Jonathan conducts an experiment to determine what solutes readily dissolve in water. He places 3 tablespoons of potting soil into one cup of water. He records his observations in 15-minute increments. After a half hour, he notices that some of the soil particles have separated and sank to the bottom. O 15 minutes 30 minutes Which term best describes the combination of soil and water?
A.Mixture
B. A solution
C. an alloy
D. an emulsion
Answers
Answer:
A. Mixture
Explanation:
Why do plant animal cells both have mitochondria?
Answers
Answer:
They both need mitochondria for cellular respiration
Explanation:
hope this helps :)
which of the following molecules has hydrogen bonding as its only intermolecular force? group of answer choices none of these choices is correct h2o ch3oh nh3 hcl
Answers
None of these choices is correct. The given molecules have intermolecular forces other than hydrogen bonding, such as dipole-dipole interactions or London dispersion forces.
Among the given choices, none of these molecules exhibit hydrogen bonding as their main keyword. Hydrogen bonding occurs when hydrogen is bonded directly to highly electronegative elements like oxygen, nitrogen, or fluorine.
H2O (water) exhibits hydrogen bonding due to the hydrogen atoms bonding with oxygen, resulting in strong intermolecular forces. CH3OH (methanol) also has hydrogen bonding because of the oxygen-hydrogen bonds.
However, NH3 (ammonia) and HCl (hydrochloric acid) have dipole-dipole interactions as their main intermolecular forces. Ammonia has a lone pair of electrons on the nitrogen atom, creating a dipole moment. Hydrochloric acid has a polar covalent bond, leading to dipole-dipole interactions.
In conclusion, while all the given molecules have intermolecular forces, hydrogen bonding is not the only intermolecular force present in any of them.
Learn more about hydrogen bonding here:
https://brainly.com/question/31139478
#SPJ11
A
is scientific knowledge established through direct observation and remains constant. Scientific knowledge can change when scientists
.
Answers
Answer:
Explanation:Scientific knowledge is knowledge in, or in connection with, any of the sciences or technology, that is accumulated by systematic study and organized by general principles. Scientific knowledge refers to a generalized body of laws and theories to explain a phenomenon or behavior of interest that are acquired using the scientific method⁴. Laws are observed patterns of phenomena or behaviors, while theories are systematic explanations of the underlying phenomenon or behavior.
Scientific knowledge is not established through direct observation alone, nor does it remain constant. Scientific knowledge can change when scientists discover new evidence, test existing hypotheses, revise existing theories, or develop new methods or technologies. Science is a dynamic and ongoing process that seeks to understand the physical world and its phenomena in a rigorous and objective way.
Find the chemical formula for aluminum oxide based on ionic
bonding from valence electrons
Answers
The chemical formula for aluminum oxide based on ionic bonding from valence electrons is Al₂O₃.
The chemical formula of a compound is a symbolic representation of its chemical composition.
Chemical formulae gives information about the elements that constitute the molecules of a compound and also about the ratio in which the atoms of these elements combine to form such molecules.
In this compound, aluminum (Al) donates three electrons to oxygen (O), resulting in the formation of Al³⁺ cations and O²⁻ anions. The ionic bond is formed between these oppositely charged ions, resulting in the formula Al₂O₃.
Learn more about Chemical Formula, here:
https://brainly.com/question/32018188
#SPJ4
Help please me this is important!!

Answers
Seven squares represent seven f-subshell orbitals with magnetic quantum number values of -3, -2, -1, 0, +1, +2, +3.
The paramagnetic nature is represented by a single electron in the seventh orbital.
This quantum number describes the spatial orientations of electrons.
What are magnetic quantum numbers?It defines the orientation of an orbital in space of a given energy(n) and shape using magnetic quantum numbers (l). It divides the subshell into orbitals made up of electrons.
It is represented by the symbol ml. Each subshell contains 2l+1 orbitals. The value of lies between -l and +l, where l is the azimuthal quantum number.
Orbitals with l=0, s-subshell, and ml = 0 = 1
P-subshell ml = -1, 0, +1 = 3 orbitals for l=1.
When l=2, the d-subshell ml = -2, -1, 0, +1, +2 = 5 orbitals.
For l=3, the f-subshell ml are -3, -2, -1, 0, +1, +2, +3 = 7 orbitals.
These orbitals are represented as squares in orbital notations of an atom, with electrons represented by upward and downward arrows.
To know more about azimuthal quantum number, visit:
https://brainly.com/question/30024510
#SPJ1
help please first person to get it right gets my old Netflix account

Answers
Answer:
Most Monarch butterflies live only for a few weeks However, some will migrate to warmer climates and survive the entire winter.
2. Briefly list and describe radiocarbon and radiopotassium
dating methods. What chemical process forms the basis of the
method? How, in general, does each work? Time frame? (10-15
sentences explanati
Answers
Radiocarbon dating, also known as carbon-14 dating, is a method used to determine the age of organic materials. It is based on the radioactive decay of the isotope carbon-14 (14C).
Living organisms constantly absorb carbon, including a small amount of carbon-14, from the atmosphere. When an organism dies, it no longer takes in carbon-14, and the existing carbon-14 begins to decay at a known rate. By measuring the ratio of carbon-14 to stable carbon isotopes (carbon-12 and carbon-13) in a sample, scientists can estimate the time that has elapsed since the organism's death. Radiocarbon dating is effective for dating materials up to about 50,000 years old.
Therefore, both radiocarbon dating and radiopotassium dating rely on the principles of radioactive decay. The decay rates of the isotopes used in these methods are well-established and constant, allowing for accurate age determinations.
For more details regarding radiopotassium dating methods, visit:
https://brainly.com/question/32267082
#SPJ4
How many molecules are in 47. 93 g sample of magnesium nitrate ? Please show The whole work
Answers
The number of molecules in 37.93g of magnesium nitrate will be : 0.2568 mol * 6.022x10^23 molecules/mol = 1.55x10^24 molecules.
To determine the number of molecules in a 37.93 gram sample of magnesium nitrate, you would need to know the molar mass of the compound. Magnesium nitrate has a molar mass of 148.31 g/mol.
we can use the formula:
Number of moles = mass (in grams) / molar mass (in g/mol)
Number of moles = 37.93 g / 148.31 g/mol = 0.2568 mol Avogadro's number (6.022x10^23) is the number of atoms, ions, or molecules in one mole of a substance. Therefore, the number of molecules in 37.93g of magnesium nitrate will be : 0.2568 mol * 6.022x10^23 molecules/mol = 1.55x10^24 molecules.
Learn more about molar mass here:
https://brainly.com/question/22997914
#SPJ4
what are homologous series
Answers
A homologous series is a family of hydrocarbons with similar chemical properties who share the same general formula. We will look at three hydrocarbon series: alkanes, alkenes and the cycloalkanes. Hydrocarbons are compounds that contain only hydrogen and carbon.
Answer:
A homologous series is a series of compounds with the same general formula, usually varying by a single parameter such as the length of a carbon chain. Compounds within a homologous series typically have a fixed set of functional groups that gives them similar chemical and physical properties.
Predict the products and balance the equation.
NaCl (aq) + KNO₂ (aq) →
Answers
Sodium chloride is produced and used in the production of polyester, paper, rubber, glass, chlorine, household bleach, soaps, detergents, and dyes.
Is salt the same as sodium chloride?Chemically speaking, salt is a combination of chloride and sodium. Actually, the element that is most harmful to your health is sodium. (Therefore, the chloride is what gives food its "salty" flavor.).
Why do doctors administer sodium chloride to patients?To replace salt and water that have been lost from your body as a result of specific conditions, sodium chloride 23.4% injection is employed (eg, hyponatremia or low salt syndrome). Additionally, it is added to IV fluids that contain carbohydrates and parenteral nutrition total (TPN).
To know more about sodium chloride visit:
https://brainly.com/question/9811771
#SPJ1
how many liters will 3.20 moles of oxygen gas occupy at STP?
Answers
Answer:
70.1 liters
Explanation:
At Standard Temperature and Pressure (STP), the conditions are defined as a temperature of 0°C (273.15 K) and a pressure of 1 atm. To calculate the volume that 3.20 moles of oxygen gas would occupy at STP, we can use the Ideal Gas Law:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Substituting the known values:
P = 1 atm
n = 3.20 moles
R = 0.0821 L·atm/mol·K
T = 273.15 K
Solving for V:
V = (nRT) / P = (3.20 moles * 0.0821 L·atm/mol·K * 273.15 K) / 1 atm = 70.1 liters
In conclusion, 3.20 moles of oxygen gas would occupy 70.1 liters at STP.
which of the following would have a higher rate of effusion than c2h2?
group of answer choices
n2
o2
cl2
ch4
co2
Answers
The gas that would have a higher rate of effusion than C2H2 (acetylene) is N2 (nitrogen gas).
Effusion refers to the process by which gas molecules escape through a small opening or a porous barrier. According to Graham's Law of Effusion, the rate of effusion of a gas is inversely proportional to the square root of its molar mass. This means that gases with lower molar masses will have higher rates of effusion.
Comparing the molar masses of the given gases:
N2: 28 g/mol
O2: 32 g/mol
Cl2: 71 g/mol
CH4: 16 g/mol
CO2: 44 g/mol
Among these gases, N2 has the lowest molar mass of 28 g/mol. Therefore, according to Graham's Law of Effusion, N2 would have a higher rate of effusion compared to C2H2.
Know more about Effusion here:
https://brainly.com/question/31076977
#SPJ11
ving things have basic needs that must be met to survive and grow. Which needs are illustrated in the picture? Check all that apply.
the need for food
the need for a place to live
the need for air
the need for water
the need for parents
ANSWER: food, air, and a place to live!!
Answers
Answer:
Explanation:
ANSWER:
the need for food, the need for air, and need for a place to live.
Live need water but here in the picture we didn't saw water; so we can't choose water;
Please help How many moles of a gas sample are in 14.0 L container at 269 K and 358 kPa? The gas constant is 8.31 L kPa/ mol K Round you answer to one decimal place and enter the number only with no units.
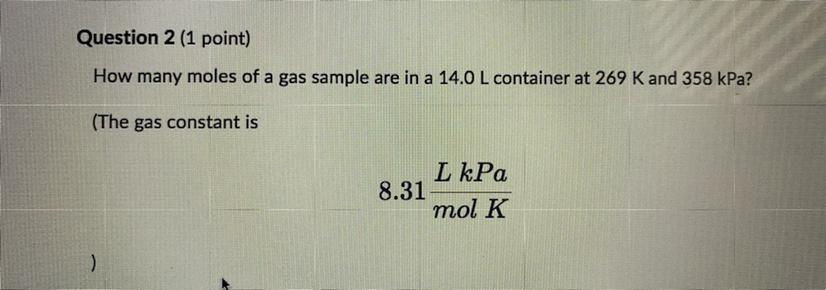
Answers
We are going to assume that the gas mentioned behaves like an ideal gas. The equation that describes the behavior of an ideal gas is as follows:
\(PV=nRT\)Where,
P is the pressure of the gas, 358kPa
V is the volume of the gas, 14.0L
R is the gas constant, 8.31 L kPa/mol K
T is the temperature of the gas, 269K
Now, we will clear the number of moles, n.
\(n=\frac{PV}{RT}\)We replace the known data:
\(\begin{gathered} n=\frac{358kPa\times14.0L}{8.31\frac{L.kPa}{mol.K}\times269K} \\ n=\frac{358\times14.0}{8.31\times269}mol \\ n=2.24mol \end{gathered}\)Answer: In the sample of gas there are 2.24 moles
100 points help is appreciated
Apply: For a strong base, the concentration of hydroxide ions [OH–] is roughly estimated to be the same as the concentration of the base. The pH of a strong base is found with the equation pH = 14 + log10[OH–]. Based on their concentrations, find the pH of each of the strong bases. Check your answers with the Gizmo.
Answer
[Ca(OH)2] = ?
pH Ca(OH)2 = ?
[NaOH] = ?
pH NaOH = ?
Answers
Litmus is an example of an indicator, a substance that changes color depending on its pH (pH is a measure of the concentration of protons, or H+ ions). In the Titration Gizmo™, you will use indicators to show how acids are neutralized by bases, and vice versa.
To begin, check that 1.00 M NaOH is selected for the Burette, Mystery HBr is selected for the Flask, and Bromthymol blue is selected for the Indicator.
Calculate: Concentration is measured by molarity (M), or moles per liter. Brackets are also used to symbolize molarity. For example, if 0.6 moles of HNO3 are dissolved in a liter of water, you would say [HNO3] = 0.6 M.
Because HNO3 is a strong acid, it dissociates almost completely in water. That means the concentration of H+ is very nearly equal to that of HNO3.What is [H+] if [HNO3] is 0.01 M? 0.01 M
The pH of a solution is equal to the negative log of H+ concentration: pH = –log[H+]
Describe: The equation for the reaction of nitric acid (HNO3) and sodium hydroxide (NaOH) is shown on the bottom right of the Gizmo.
Measure: A titration can be used to determine the concentration of an acid or base by measuring the amount of a solution with a known concentration, called the titrant, which reacts completely with a solution of unknown concentration, called the analyte. The point at which this occurs is called the equivalence point.
Explain: A titration curve is a graph of pH vs. volume of titrant. The graph at right shows a typical titration curve for the titration of a strong acid by a strong base. (A strong base is one that has relatively high dissociation in water.)
According to theBrønsted-Lowry definition, an acid is a substance that is capable of donating a proton to another substance. A base is a substance that accepts protons. When an acid and a base are combined, the acid is neutralized as the base accepts the protons produced by the acid.One way to determine if a solution is acidic or basic is to use litmus paper, as shown above. There are two types of litmus papers: red and blue.How does litmus paper indicate an acid? Both strips turn red.
contrast longitudinal and transverse waves by describing the particle movement in sound and water waves :
Answers
Answer: In a transverse wave, the particles of the medium move perpendicular to the wave's direction of travel. Transverse waves are characterized by peaks and valleys, called crests and troughs. In a longitudinal wave, the particles of the medium move parallel to the wave's direction of travel.
Explanation:
In case of transverse wave, the particles move perpendicular to the wave's direction and it is characterized by peaks and valleys, called crests and troughs. longitudinal wave made up of rarefactions and compressions.
What are the difference between longitudinal and transverse waves ?A disturbances that propagates energy from one place to another place without the involvement of transporting matter called as wave.
In a longitudinal type of wave, the medium moves in the same direction, particles move from left to right.
It moves in dimension, cannot be polarized or aligned, produced in gas, liquid or solid, example is earthquake P wave.
In a transverse type of wave, the medium moves perpendicular to the wave's the direction, the particles move up and down.
It directs in two dimensions, can be polarized or aligned, produced in solid and liquid’s surface, example is Earthquake S wave.
For more details regarding longitudinal wave, here
https://brainly.com/question/3513157
#SPJ2
When tin (IV) oxide is heated with carbon, the element tin can be extracted. Balance the equation and then interpret it in terms of particles, moles and mass. Show that the law of conservation of mass is observed.
__SnO2 (s) + __C (s) → __Sn (l) + __ CO (g)
Answers
The balanced equation for the reaction between tin (IV) oxide and carbon is:
SnO2 (s) + 2C (s) → Sn (l) + 2CO (g)
This equation indicates that one mole of tin (IV) oxide reacts with two moles of carbon to produce one mole of tin and two moles of carbon monoxide. The molar mass of SnO2 is 150.71 g/mol, while the molar mass of carbon is 12.01 g/mol. Therefore, the mass of 1 mole of SnO2 is 150.71 g and the mass of 2 moles of carbon is 24.02 g.
According to the law of conservation of mass, the total mass of the reactants should be equal to the total mass of the products. In this case, the total mass of the reactants is:
150.71 g + 24.02 g = 174.73 g
The total mass of the products is:
118.69 g + 56.04 g = 174.73 g
As we can see, the mass of the reactants is equal to the mass of the products, which confirms that the law of conservation of mass is observed.
In terms of particles, the equation indicates that one molecule of SnO2 reacts with two molecules of carbon to produce one atom of tin and two molecules of carbon monoxide. The mass of the products is less than the mass of the reactants due to the production of carbon monoxide gas, which has a lower mass than solid carbon and tin oxide. This reaction demonstrates the importance of conservation in chemical reactions, as it shows how mass is neither created nor destroyed, but rather transformed into different forms.
To know more about carbon visit:
https://brainly.com/question/13046593
#SPJ11
->>
16) Which of the following single-replacement reactions will result in NO REACTION?
A. Na(s) + Mg(NO3)2(aq)
B. Na(s) + Al(NO3)2(aq) ->
C. Na(s) + Cu(NO3)2(aq) ->>
D. Na(s) + Fe(NO3)2(aq) →>>
E. Na(s) + Ba(NO3)2(aq)