Answers
Answer:
An acute triangle (or acute-angled triangle) is a triangle with three acute angles (less than 90°).
Explanation:
hope this helps
Related Questions
for every 1lb you lose do to sweating you should consume ___ oz of water.
Answers
Answer:
For every pound lost, replace it with 16 to 20 ounces of fluid
What of the following is made out of atoms? (HINT: vocabulary word "atom" or "matter") * 1 Air
2 Light
3 Energy
4 Sound
Answers
The correct IUPAC name for the structure shown is
A)
ethylmethylamine.
B)
methylamine.
C)
ethylamine.
D)
ethylmethylhydridoamine.

Answers
Answer:
A
Explanation:
it has a methyl group, ethyl group and amine group
The correct IUPAC name for the structure shown in the provided image is "ethylamine." The structure consists of a central nitrogen atom bonded to two carbon atoms.
The correct IUPAC name for the structure shown in the provided image is "ethylamine." The structure consists of a central nitrogen atom bonded to two carbon atoms. According to the IUPAC naming rules, the longest carbon chain is selected as the parent chain, which in this case consists of two carbon atoms. The substituent attached to the parent chain is an ethyl group, denoted as "C2H5". The amine functional group, which consists of the nitrogen atom, is named as "amine". Since there is only one amine group attached to the carbon chain, it is referred to as "ethylamine." Therefore, option C) "ethylamine" is the correct IUPAC name for the given structure.
For more question on IUPAC
https://brainly.com/question/28872356
#SPJ11
What is photosynthesis??...
Answers
the process plants use sunlight to synthesize food
Answer:
It is when green plants and other organisms transform light energy into chemical energy.
the pressure of a sample of gas in a 13 l container is .2 atm. what is the new volume if the pressure changes to 1 atm
Answers
The pressure of a sample of gas in a 13 l container is .2 atm. 2.6L is the new volume if the pressure changes to 1 atm
The area that any three-dimensional solid occupies is known as its volume. These solids can take the form of a cube, cuboid, cone, cylinder, or sphere. Various forms have various volumes. We have examined a variety of solids and forms in 3D geometry, including cubes, cuboids, cylinders, cones, etc.
P1×V1 = P2×V2
0.2×13 = 1×V2
V2= 2.6L
To know more about volume, here:
https://brainly.com/question/28058531
#SPJ1
From what carboxylic acid derivative can urea most easily be prepared?carbonic acid, formyl chloride, phosgene, carbamic acid, or diurea
Answers
From Phosgene, the carboxylic acid derivative can urea most easily be prepared.
Phosgene is the organic chemical compound with the formula COCl2. It is the carboxylic acid derivative. It is a toxic, colorless gas. in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block for the production of precursors of polyurethanes and polycarbonate plastics. Phosgene is extremely poisonous. It was a highly potent pulmonary irritant and quickly filled enemy trenches due to it being a heavy gas. It is a colorless gas with a suffocating odor like musty hay. Exposure to phosgene may cause irritation to the eyes, dry burning throat, vomiting, cough, foamy sputum, breathing difficulty, and chest pain.
To learn more about Phosgene please visit:
https://brainly.com/question/11477415
#SPJ4
Describe what the electrons are doing in an atom to cause the flame
test color. Include the words excited state, ground state, and quantum
in your explanation?
Answers
To learn more about flame test visit:
https://brainly.com/question/6357832
#SPJ9
Methyl pentanoate condensed structural formula
Answers
Answer:
C6H12O2 is the formula for Methyl pentanoate
Which of the following solutions is acidic?
A. pH 7
B. pH 5
C. pH 14
D. pH 10
need answer quick
Answers
You are given 1.091 grams of a white powder and told that it is a mixture of potassium carbonate and sodium carbonate. You are asked to determine the percent composition by mass of the sample. You add some of the sample to 10.00 mL of 0.8903 M nitric acid until you reach the equivalence point. When you have added enough carbonate to completely react with the acid, you reweigh your sample and find that the mass is 0.573 g. Calculate the mass of the sample that reacted with the nitric acid. Calculate the moles of nitric acid that reacted with the sample.
Answers
The sample of white powder contains 47.1% K2CO3 and 0.39% Na2CO3.
Molar mass of sodium carbonate = 106 g/mol
Molar mass of potassium carbonate = 138 g/mol
Number of moles of HNO3 = 10/1000 L × 0.8903 M = 0.008903 moles
Mass of HNO3 = 0.008903 moles × 63 g/mol = 0.56 g
Mass of sample added = 1.091 g
Mass of sample left over = 0.573 g
Mass of sample reacted = 1.091 g - 0.573 g = 0.518 g
The reacted sample contains xg of Na2CO3 and (0.518 - x) g K2CO3.
Na2CO3 + 2HNO3 --> 2NaNO3 + CO2 + H2O
106g of Na2CO3 reacts with 126g of HNO3
x g of Na2CO3 reacts with (126 × x/106)g of HNO3
K2CO3 + 2HNO3 --> 2KNO3 + CO2 + H2O
138 g of K2CO3 reacts with 126 g of HNO3
(0.518 - x) g of K2CO3 reacts with [(0.518 - x) × 126/138] g
Total mass of HNO3 used;
1.19x + 0.47 + 0.91x = 0.56
2.1x + 0.47 = 0.56
2.1x = 0.56 - 0.47
2.1x = 0.09
x = 0.09/2.1
x = 0.0043 g
Mass of K2CO3 = (0.518 - x) g = 0.518 - 0.0043 = 0.5137 g
Mass percent of K2CO3 = 0.5137 g/ 1.091 g × 100/1 = 47.1%
Mass percent of Na2CO3 = 0.0043/1.091 g × 100/1 = 0.39%
Learn more: https://brainly.com/question/9743981
FC1O₂(g) → FC10(g) + O(g)
The first-order decomposition of FC1O₂ (g) is represented by the equation above. At a certain temperature, the partial pressure of FC1O2(g) in a sealed vessel falls from 0.080 atm to 0.010 atm over 48
minutes.
What is the half-life of the decomposition reaction?
Answers
The half-life of a first-order reaction is given by the following equation:
t(1/2) = ln(2)/k
where t(1/2) is the half-life and k is the rate constant.
From the given information, we know that the partial pressure of FC1O2(g) falls from 0.080 atm to 0.010 atm over 48 minutes. We can use this information to calculate the rate constant as follows:
ln([FC1O2]t/[FC1O2]0) = -kt
where [FC1O2]t is the concentration (in atm) at time t, [FC1O2]0 is the initial concentration (in atm), and k is the rate constant.
Substituting the given values, we get:
ln(0.010/0.080) = -k(48)
Solving for k, we get:
k = 0.1225 min^-1
Now, we can use the equation for the half-life to calculate the answer:
t(1/2) = ln(2)/k
t(1/2) = ln(2)/0.1225
t(1/2) = 5.66 min (rounded to two significant figures)
Therefore, the half-life of the decomposition reaction is 5.66 minutes.
The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2
in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
Answers
d.2.93m.CaCl2 is present in the solution with a 2.93m concentration.
The boiling point of a solution is directly related to its concentration. The boiling point elevation of a solution, ΔTb, is equal to the product of the van't Hoff factor (i) and the molality of the solution (m).The quantity of moles of solute per kilogramme of solvent is known as molality.
Therefore, we can solve for the molality of the solution using the following equation:
ΔTb
\(= i *m\\105.3\°C= i * m\\\)
\(m =\frac{ 105.3 \°C }{i}\)
Assuming an ideal van't Hoff factor for CaCl2 (i = 2), the molality of the solution is:
\(m =\frac{ 105.3 \°C }{ 2}\\m = 52.65 m = 52.65 mol/kg\)
The concentration of CaCl2 in the solution is then:
\(C = m * Kb\\C = 52.65 mol/kg * 0.512 \°C/m\\C = 2.93 mol/kg\)
Therefore,The concentration of CaCl2 in the solution is 2.93m.
learn more about boiling point refer:brainly.com/question/24168079
#SPJ1
complete question:The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2 in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
a.3.45m
b.4.40m
c.8.79m
d.2.93m
Potassium Phthalate, KC8H604, is a main source of whistle fuel for many fireworks. A standard firework had 25g of potassium phthalate. Calculate the following:
The molar mass of the compound.
How many molecules of fuel there are
The number of moles of Carbon
Answers
Answer:
Explanation:
The given compound is:
KC₈H₆O₄
Mass of the compound = 25g
Molar mass of the compound
KC₈H₆O₄
Atomic mass of K = 39
C = 12
H = 1
O = 16
Molar mass of the compound = 39 + 8(12) + 6 (1) + 4(16)
= 205g/mol
Number of molecules of fuel there in:
Find the number of moles then convert this to number of molecules;
Number of moles = \(\frac{mass}{molar mass}\)
Number of moles = \(\frac{25}{205}\) = 0.12mol
1 mole of a compound contains 6.02 x 10²³ molecules
0.12 mole of the phthalate will contain 0.12 x 6.02 x 10²³ =
7.34 x 10²²molecules
Number of moles of Carbon
1 mole of the phthalate contains 8 mole of carbon
0.12 mole of the phthalate will contain 0.12 x 8 = 0.96mole of carbon
Hydrogen reacts with oxygen according to the balanced equation
2H₂ (g) + O2(g) → 2H₂O(g). If X is the number of molecules of H₂ which react,
then the number of O2 molecules reacting is
Answers
Answer:
x/2
Explanation:
X = 2 molecules of H2
For 2 molecules of H2, there's only 1 molecule of O2. Meaning, there's twice the amount of H2, so O2 = x/2 molecules.
I hope I'm understanding this question right.
When the equation
Ca3(PO4)2+HNO3→Ca(NO3)2+H3PO4
is balanced, the smallest whole number coefficient for HNO3 is
Answers
Answer:
The least whole number coefficient for HNO₃ is 6
Explanation:
The chemical equation above is the reaction between calcium orthophosphate and nitric acid.
To balance a chemical equation, we have to consider law of conservation of matter which states that matter can neither be created nor destroyed.
What this law implies is that, whatever we have at the reactant side must be equal to whatever is obtainable at the product side.
The above equation is
Ca₃(PO₄)₂ + HNO₃ → Ca(NO₃)₂ + H₃PO₄
To balance the equation, we'll have to check the number of atoms at each side and possibly balance the equation with the number of moles.
The balanced equation is
Ca₃(PO₄)₂ + 6HNO₃ → 3Ca(NO₃)₂ + 2H₃PO₄
From the balanced equation above, we can see that the number of calcium (Ca), Phosphorus (P), Oxygen(O), Nitrogen(N) and hydrogen (H) are balanced at both sides of the equation.
The least number coefficient for HNO₃ is 6
If 46 students take a chemistry exam and 85% of them past the exam how many students do not pass the exam
Answers
7 students did not pass the exam
if there are 46 students who took the chemistry exam and 85 % of the students pass the exam then the remaining 15% must have failed or did not pass the exam. Now we convert the percentage of students who failed to the numerical value.
now 15% of 46 = 46×15/100 = 6.9
but obviously, there cannot be 6.9 students. So the answer is that 7 students did not pass the chemistry exam.
Learn more about percentage:
https://brainly.com/question/18941982?referrer=searchResults
Consider the insoluble compound nickel(II) hydroxide , Ni(OH)2 . The nickel ion also forms a complex with cyanide ions . Write a balanced net ionic equation to show why the solubility of Ni(OH)2 (s) increases in the presence of cyanide ions and calculate the equilibrium constant for this reaction. For Ni(CN)42- , Kf = 1.0×1031 . Use the pull-down boxes to specify states such as (aq) or (s).
Answers
Answer: Equilibrium constant for this reaction is \(2.8 \times 10^{15}\).
Explanation:
Chemical reaction equation for the formation of nickel cyanide complex is as follows.
\(Ni(OH)_{2}(s) + 4CN^{-}(aq) \rightleftharpoons [Ni(CN)_{4}^{2-}](aq) + 2OH^{-}(aq)\)
We know that,
K = \(K_{f} \times K_{sp}\)
We are given that, \(K_{f} = 1.0 \times 10^{31}\)
and, \(K_{sp} = 2.8 \times 10^{-16}\)
Hence, we will calculate the value of K as follows.
K = \(K_{f} \times K_{sp}\)
K = \((1.0 \times 10^{31}) \times (2.8 \times 10^{-16})\)
= \(2.8 \times 10^{15}\)
Thus, we can conclude that equilibrium constant for this reaction is \(2.8 \times 10^{15}\).
which of the following are the correct formulas for potassium oxide and calcium oxide respectively
Answers
Explanation:
potassium oxide is K2O
calcium oxide is CaO
calcium oxide is used to make glass
potassium oxide is used in fertilizer
SOMEONE PLEASE HELP!!!!!!!! An element with 70 protons and a mass of 170 would be considered:
a. stable
b. radioactive
c. nonexistent
Answers
Answer:
Explanation:
An element with 70 protons and a mass of 170 would be considered radioactive.
In general, an element with an atomic number (number of protons) greater than 82 tends to be radioactive. Since the element in question has 70 protons, which is less than 82, it does not fall into the category of naturally radioactive elements. However, it is important to note that the stability of an element also depends on the balance between protons and neutrons in the nucleus. Without information about the number of neutrons in the nucleus, we cannot determine the stability of this specific element definitively.
Which substance would have the highest melting point, based on electronegativity?
H2O
H2S
H2Te
H2Se
Answers
Answer:
H₂O
Explanation:
Based electronegativity, water H₂O will have the higher melting point from the given choices. The binding force between hydrogen and oxygen is greater than for the others.
In group 6, oxygen has the highest electronegativity. It pulls the shared electron closer in the bond. The high electronegativity between hydrogen and oxygen causes the elevated melting point between the two species.Answer:
H2O
Explanation:
hoffe das hilfts
Calculate the cell potential, Ecell, for the following reaction at 298k.
Co(s)+2Ag+(0.010M)=Co+2(0.015M)+2 Ag(s)
Answers
To calculate the cell potential, Ecell, for the given reaction at 298K, we need to use the Nernst equation. The Nernst equation relates the cell potential to the standard cell potential, temperature, and the concentrations of the reactants and products. The Nernst equation is given as follows:
Ecell = E°cell - (RT/nF) ln(Q)
where,
Ecell = cell potential
E°cell = standard cell potential
R = gas constant (8.314 J/K.mol)
T = temperature (298 K)
n = number of electrons transferred in the balanced redox reaction
F = Faraday constant (96,485 C/mol)
Q = reaction quotient
The given reaction is a redox reaction, which involves the transfer of two electrons from Co to Ag+. The balanced half-reactions are as follows:
Co(s) → Co2+(aq) + 2 e-
Ag+(aq) + e- → Ag(s)
The standard reduction potentials for these half-reactions are:
Co2+(aq) + 2 e- → Co(s) E°red = -0.28 V
Ag+(aq) + e- → Ag(s) E°red = +0.80 V
The overall standard cell potential can be calculated by subtracting the standard reduction potential of the anode from that of the cathode:
E°cell = E°red,cathode - E°red,anode
= +0.80 V - (-0.28 V)
= +1.08 V
Now we need to calculate the reaction quotient Q using the concentrations of the reactants and products. According to the given information, [Ag+] = 0.010 M and [Co2+] = 0.015 M.
Q = ([Co2+][Ag+]^2)/([Ag+]^2)
= ([0.015][0.010]^2)/([0.010]^2)
= 0.015 M
Substituting the values in the Nernst equation, we get:
Ecell = E°cell - (RT/nF) ln(Q)
= 1.08 - (8.314 x 298 / (2 x 96485)) ln(0.015)
= 0.829 V
Therefore, the cell potential, Ecell, for the given reaction at 298K is 0.829 V.
Determine the energy change associated with the transition from n = 4 to n = 1 in the hydrogen atom.Group of answer choices:3.27 × 10-17 J4.62 × 10-19 J2.04 × 10-18 J1.64 × 10-18 J6.54 × 10-18 J
Answers
• The energy change associated with the transition from n = 4 to n = 1 in the hydrogen atom can be calculated as follows :,
∆E = RH ( 1/nf -1/ni){where nf = 1 andni = 4 ( (n final) is the level you end up at, and (n initial) is the one you started from.)}
So, ∆E = RH ( 1/nf -1/ni) = (-2.18 * 10^-18 J)( 1/4 -1/1)
= 2.04375 X 10^-18 J of energy is emitted.
Therefore , option C is the correct answer.When 7.59 grams of sodium hydroxide (NaOH) are dissolved in 80.0 grams of water at 25.0 °C in an insulated container, the temperature of the water increases to 48.0 °C. Assuming that the specific heat of the solution is 4.184 J/(g °C) and that no heat is gained or lost by the container, what is the ∆H of solution of NaOH in kJ/mol?
Answers
The ∆H of solution of NaOH is 46.8 kJ/mol.
First, we need to calculate the amount of heat absorbed by the solution:
q = m × c × ∆T
where q is the heat absorbed (in Joules), m is the mass of the solution (in grams), c is the specific heat capacity of the solution (in J/(g °C)), and ∆T is the change in temperature (in °C).
In this case, the mass of the solution is the sum of the mass of NaOH and the mass of water:
m = 7.59 g + 80.0 g = 87.59 g
The change in temperature is:
∆T = 48.0 °C - 25.0 °C = 23.0 °C
Substituting the values, we get:
q = 87.59 g × 4.184 J/(g °C) × 23.0 °C = 8,878 J
Next, we need to convert the heat absorbed into the enthalpy change of solution (∆H). The enthalpy change of solution is the heat absorbed per mole of solute. The number of moles of NaOH is:
n = m/M
where M is the molar mass of NaOH, which is 40.00 g/mol.
n = 7.59 g / 40.00 g/mol = 0.1898 mol
Therefore, the enthalpy change of solution is:
∆H = q/n = 8,878 J / 0.1898 mol = 46,780 J/mol = 46.78 kJ/mol
The H of a NaOH solution, rounded to three significant numbers, is 46.8 kJ/mol.
To know more about the Temperature, here
https://brainly.com/question/30411639
#SPJ1
The melting points and boiling points of four elements are shown.
element
melting
point/°C
boiling
point/°C
3
-7
60
х
-101
-34
Y
114
184
z
39
688
slid
In which elements do the particles vibrate about fixed positions at 0°C?
А
Wand X
B
W and Z
с
X and Y
D
Y and Z

Answers
Answer:
6AM and the other three of them have to be the best team code in nepal for a while now I will not happen again which is a great opportunity for the club and I think it will not happen to him but he will send him to do the same with a club like this one event and he is the only player who is going through a tough game and we are not.....
balanced equation for beta decay of iodine-138
Answers
Answer:
Explanation:
Please take a look at the attached picture about the general formula of beta decay and the answer.
beta decay is an atom breaking down into a new atom of another element which the atomic number is 1 larger than the original, and one electron (β particle). The mass no. of the new atom remains same as before.
138 is iodine's mass number, the atomic number of iodine is 53, referred from a periodic table. The new atom formed will be Xe, which has an atomic no. of 54.
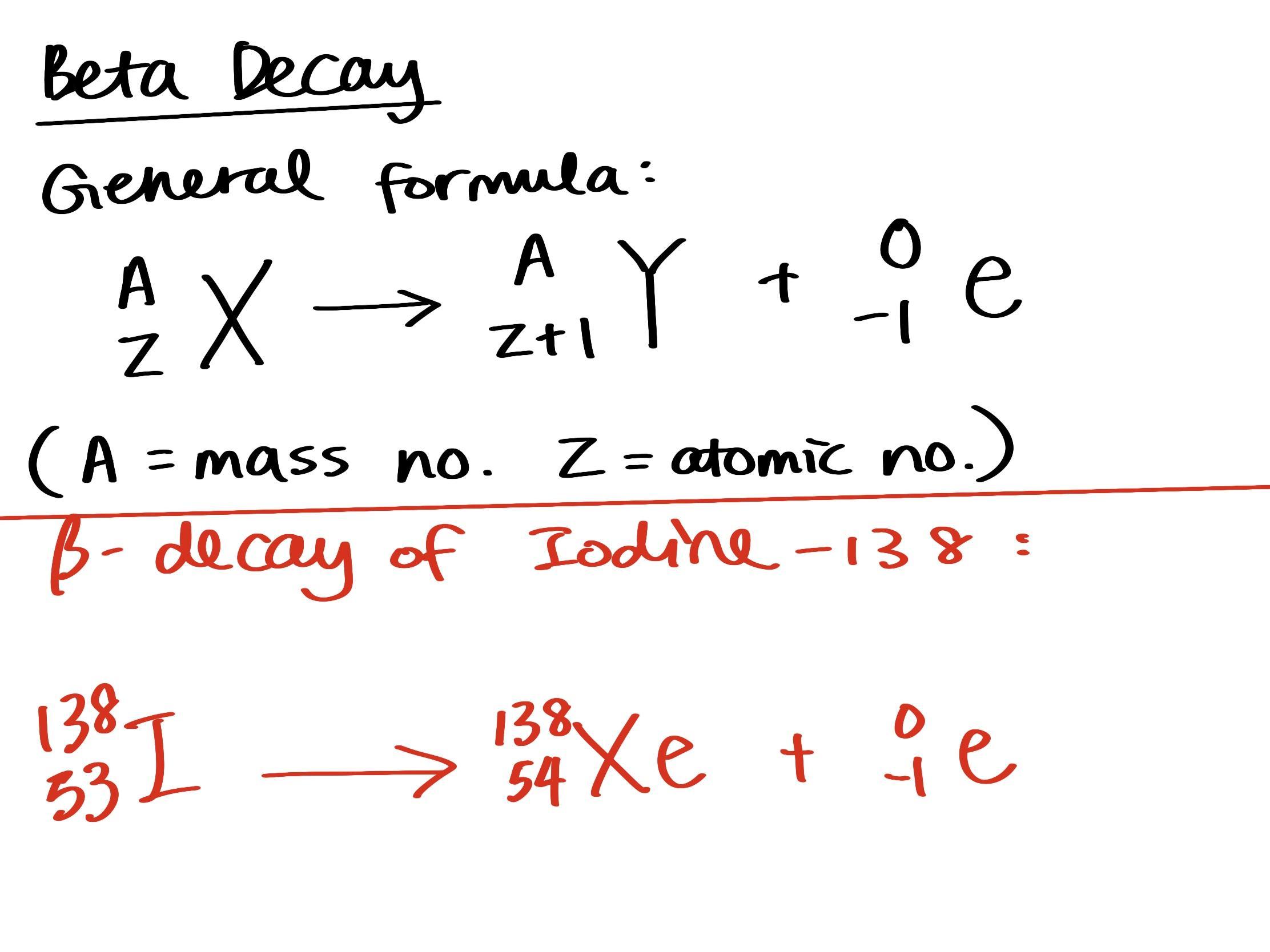
How many significant digits should be used to report the answer to each of the following calculations? (2.75518 + 9.01 + 3.3349) / (2.1)
Answers
Answer:
2
Explanation:
You write your answer with the same number of significant figures as the number with the smallest amount of figures, in this case, that number is 2.1 so your answer should be written with 2 significant figures.
[OH﹘] = 6.5 x 10-5Mfind the pH and pOH
Answers
pH = 9.81
pOH = 4.19
Explanations:The formula for calculating the pOH of a solution is given as:
\(pOH=-log[OH^-]\)Given the following parameters
\([OH^-]=6.5\times10^{-5}M\)Substitute
\(\begin{gathered} pOH=-log(6.5\times10^{-5}) \\ pOH=-(-4.19) \\ pOH=4.19 \end{gathered}\)Determine the pH of the solution
\(\begin{gathered} pH+pOH=14 \\ pH=14-pOH \\ pH=14-4.19 \\ pH=9.81 \end{gathered}\)Therefore the pH and pOH are 9.81 and 4.19 respectively
The boiling point of a substance is tested. After 10 tests, the result is given as 37+/−3°C. Which conclusion can be drawn from this result?
A- The scientists do not need to collect more data because they have narrowed down the range of the results.
B- The scientists should not report these results until they have the exact number.
C- The actual boiling point is either 34°C or 40°C.
D- The actual boiling point is probably between 34°C and 40°C.
Answers
From the data obtained from the tests, the actual boiling point lies between 34°C and 40°C.
The boiling point of a substance is the temperature at which the pressure of the substance becomes equal to atmospheric pressure. Pure substances have a sharp boiling point while impure substances boil over a temperature range.
In this case, the boiling point of the substance after 10 tests is obtained as 37+/−3°C. This implies that the actual boiling point lies between 34°C and 40°C.
Learn more: https://brainly.com/question/8646601
A person has a 336.33 mL sample of gas at a pressure of 25.72 kPa. If the person increases the volume to 1.18L, what will the new pressure of the gas be in kPa?
Answers
Answer;
New Pressure of Gas = 7.33 kPa
Explanation:
Given the initial and final volume occupied by a sample of a gas at constant temperature, we want to get the final pressure of the gas given the initial pressure value
To answer this, we look for the gas law that links voume and pressure at constant temperature
The gas law here is Boyle's law
It states that volume and pressure are inversely proportional. So we would expect a volume decrease where there is a pressure increase and vice versa
Mathematically, we have this as:
\(P_iV_i=P_fV_f\)where i represents the initial values and f represents the final values
What we are looking for is the final pressure so, we rewrite the formula above as follows:
\(P_f\text{ = }\frac{P_iV_i}{V_f}\)Lastly, we go on to substitute the given values
Let us have a look at the volume units
It must be understood that we should have same unit
Since 1000 mL = 1L
Then 336.33 mL = 0.33633 L
Finally, we substitute as follows:
\(P_f\text{ = }\frac{25.72\times0.33633}{1.18}\text{ = 7.33 kPa}\)Brainly placed a thermometer in a jar of hot water. The red level immediately went up. Which is the best answer? 1.) Hot water pushed the red liquid up and out of its way as water entered the thermometer. 2.) mass of the red liquid increased so it takes up more space which makes it go the thermometer. Density of the red liquid does not change 3.) molecules of the red liquid gain. Thermal energy from the hot water causes them to expand up the thermometer as they become less dense.
Answers
The reason the red level immediately went up when the thermometer was placed in the jar of hot water was 3.) molecules of the red liquid gain. Thermal energy from the hot water causes them to expand up the thermometer as they become less dense.
How does a thermometer work ?A glass tube filled with mercury that expands or contracts as the temperature changes serves as the medium through which a thermometer measures temperature. The tube and bulb are so small that the mercury can reach the temperature of what they are measuring extremely quickly.
The mercury molecules in the red liquid, which receive thermal energy from the hot water, expand up the thermometer as their density decreases.
Find out more on thermometers at https://brainly.com/question/17770038
#SPJ1