(a) What behavior distinguishes paramagnetic and diamagnetic substances?
Answers
Substance's attraction to external magnetic field i.e., their magnetic property/behavior distinguishes paramagnetic substance from diamagnetic substance.
What is paramagnetic and diamagnetic substances?A diamagnetic substance is one whose atoms have no permanent magnetic dipole moment. They are slightly repelled by a magnetic field and do not retain the magnetic properties when the external field is removed
The examples of diamagnetic materials are copper, gold, antimony, silver, lead and hydrogen.
Paramagnetic materials are those materials that are weakly attracted by the external magnetic field. They are slightly attracted by a magnetic field and do not retain the magnetic properties when the external field is removed.
Examples of paramagnetic materials are aluminium, sodium and calcium.
To learn more about magnetism, refer https://brainly.com/question/18088490
#SPJ4
Related Questions
What is the molar mass of Platinum (I) Sulfate
Answers
Answer:
196+32+16x4 = 292 g ans.
Explanation:
Write the cell notation for an electrochemical cell consisting of an anode where Pb (s) is oxidized to Pb2 (aq) and a cathode where Fe3 (aq) is reduced to Fe2 (aq) at a platinum electrode . Assume all aqueous solutions have a concentration of 1 mol/L and gases have a pressure of 1 bar.
Answers
The cell notation for the electrochemical cell with a Pb(s) anode oxidized to Pb²⁺(aq) and a platinum electrode cathode where Fe³⁺(aq) is reduced to Fe²⁺(aq) is:
Pb(s)|Pb²⁺(aq, 1M)||Fe³⁺(aq, 1M), Fe²⁺(aq, 1M)|Pt(s)
The cell notation for the given electrochemical cell can be represented as follows:
Pb(s)|Pb²⁺(aq, 1M)||Fe³⁺(aq, 1M), Fe²⁺(aq, 1M)|Pt(s)
In this notation, the single vertical line "|" represents a phase boundary, while the double vertical lines "||" represent the salt bridge that connects the two half-cells. The anode (where oxidation occurs) is written on the left, and the cathode (where reduction occurs) is written on the right.
For more such questions on cell notation, click on:
https://brainly.com/question/17218591
#SPJ11
What is the formula for ammonium sulfide? Capitalization and punctuation count. ammonium sulfide (NH4)2S I How many hydrogen atoms are in 2.50 mol of ammonium sulfide? H atoms x100
Answers
The formula for ammonium sulfide is (NH4)2S. There are 20.00 hydrogen atoms in 2.50 mol of ammonium sulfide.
To determine the number of hydrogen (H) atoms in 2.50 mol of ammonium sulfide, we need to consider the stoichiometry of the compound. From the formula (NH4)2S, we can see that each ammonium ion (NH4+) contains four hydrogen atoms.
So, in 1 mole of ammonium sulfide, we have 2 moles of ammonium ions, which means there are 2 * 4 = 8 hydrogen atoms.
To find the number of hydrogen atoms in 2.50 mol of ammonium sulfide, we can use the following conversion:
Number of hydrogen atoms = (2.50 mol) * (8 hydrogen atoms/1 mol)
Number of hydrogen atoms = 20.00
Therefore, there are 20.00 hydrogen atoms in 2.50 mol of ammonium sulfide.
learn more about "ammonium sulfide":- https://brainly.com/question/5523357
#SPJ11
1. If a cell has waste it needs to store, it will store it:
Answers
What type pf asexual reproduction produces the most offspring?
Binary Fission
Budding
Sporulation
Answers
thats kinda hot not even gon'
equation
4 is number 4
#13 Using equation 4, calculate the buffer capacity of water = M per unit pH change Answer format: Number: Round to: 4 decimal places. Show Hint √0 % #14 Please show your work for this answer using
Answers
Equation 4 is given as:pH = pKa + log ([A-]/[HA])Where pH is the negative log of the hydrogen ion concentration and pKa is the negative log of the acid dissociation constant. [A-] is the concentration of the conjugate base and [HA] is the concentration of the acid.
Using equation 4, the buffer capacity of water can be calculated using the following formula:
β = dC/dpH
where β is the buffer capacity, dC is the change in concentration of the acid or the base, and dpH is the change in pH per unit concentration.
To calculate the buffer capacity of water, we need to know the acid dissociation constant (Ka) of water. The Ka of water is 1.0 x 10-14 M at 25°C.
Therefore, the pKa of water is 14.0 at 25°C.We can assume that the concentration of water is constant, so the change in concentration of water (dC) is zero. Therefore,β = dC/dpH = 0/dpH = 0The buffer capacity of water is zero because water cannot act as a buffer since it is a neutral substance with no acid or base properties.Showing the work for Answer 14:β = dC/dpHwhere dC = 0 (since the concentration of water is constant) and dpH = 1Therefore,β = 0/1 = 0M/pHThe buffer capacity of water is zero M/pH.
To know more about dissociation constant visit:-
https://brainly.com/question/32993267
#SPJ11
I'm just straight confused by it

Answers
Answer:
B
Explanation:
(Usually give an explanation, but doing an exam, so just trust)
Question 7
What causes metallic bonds to form?
O The attraction between the delocalized electrons and the metal's anions
O The attraction between the delocalized electrons and the metal's valence electrons
O The attraction between the delocalized electrons and the metal's atoms
O The attraction between the delocalized electrons and the metal's cations
< Previous
N
Answers
Answer:
D.) The attraction between the delocalized electrons and the metal's cations
Explanation:
Metallic bonds form between free-floating valence electrons (delocalized electrons) and positively charged metal ions (cations). The negative charge from the electrons and the positive charge from the ions are what form the attraction.
I just want u to check if it’s correct or not no need for explanations
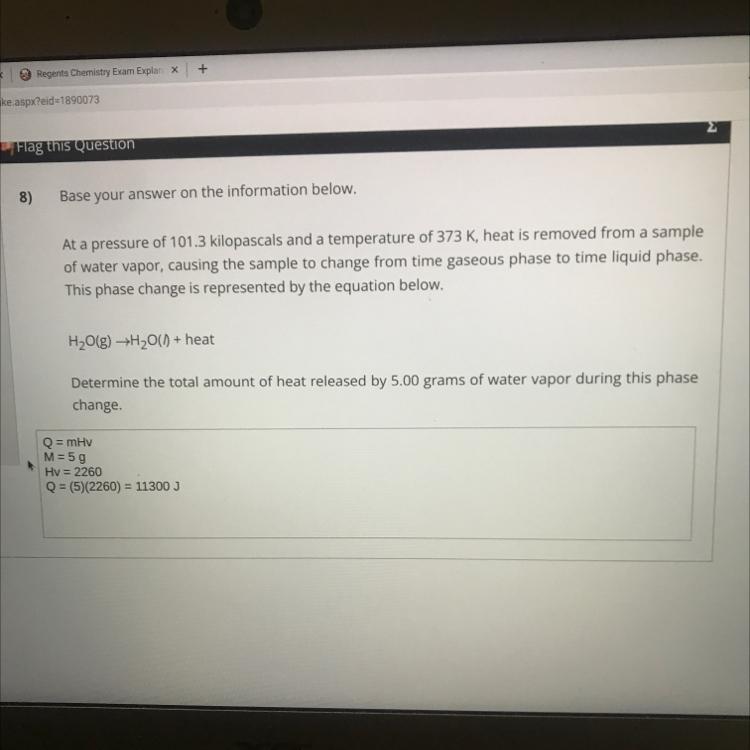
Answers
Given mass = 5g
Heat of vapour = 2260
Heat released during conversion of steam = m * C = 5 * 2260
= 11300J
Your calculations are correct.
72.A 25.5 mL aliquot of HCl (aq) of unknown concentration was titrated with 0.113 M NaOH (aq). It took 51.2 mL of the base to reach the endpoint of the titration. The concentration (M) of the acid was __________.
Answers
Given data: Volume of HCl = 25.5 mL Volume of NaOH = 51.2 mL Concentration of NaOH = 0.113 M. We need to find the concentration of HCl. Let's calculate the number of moles of NaOH present in 51.2 mL of the solution using the formula: Moles of solute (NaOH) = concentration (M) × volume (L)Moles of NaOH = 0.113 M × 51.2 mL / 1000 mL= 0.0057976 mol
Now we know that according to the balanced chemical equation for the reaction between NaOH and HCl: HCl + NaOH → NaCl + H2OOne mole of NaOH reacts with one mole of HCl. So, the number of moles of HCl is also 0.0057976 mol.Let's calculate the concentration of HCl. Concentration (M) of HCl = Moles of HCl / Volume of HCl (in L)Volume of HCl = 25.5 mL = 25.5 / 1000 L= 0.0255 LMoles of HCl = 0.0057976 mol Concentration of HCl = 0.0057976 mol / 0.0255 L= 0.227 M Therefore, the concentration (M) of the HCl solution is 0.227 M.
To know more about Moles of solute visit
https://brainly.com/question/31039725
#SPJ11
In which type of atom would radioactive decay most likely occur?
Group of answer choices
Radioactive decay will most likely occur in an atom with 26 protons.
Radioactive decay will most likely occur in atoms that have gained or lost electrons to form charged ions.
Radioactive decay will most likely occur in an atom that has more protons than electrons.
Radioactive decay will most likely occur in an atom with an unstable number of protons and neutrons in its nucleus.
Answers
Answer:
4
Explanation:
Here is a model of the sun earth system at a certain point in earths orbit around the sun based on the model which statement best explains my point is is experiencing summer
Answers
Answer:
the earth is tilted towards the sun
Explanation:
In the context, there is a sun and earth system and at a certain point when the earth is moving in its orbit around he sun, some point on the earth experiences the summers season. This is because at this point of the earths revolution, the point on the earth is facing the sun and is also tilted towards the sun. So at this point the sun rays fall directly at the surface. While the place which is tilted away from the sun, experiences winter season.
Answer:
Earth's northern hemisphere is tilted toward the Sun.
How does thermal energy flow between a hot or cold pack and the atmosphere?
Answers
Answer:
Heat is the transfer of energy. During energy transfer, the energy moves from the hotter object to the colder object. This means that the hotter object will cool down and the colder object will warm up. The energy transfer will continue until both objects are at the same temperature.
Explanation:
Taking two positively charged objects and holding them close to one another creates a system with electric potential energy
One way to lower the electric potential energy in a system like this would be to
A increase the distance between the two charges.
B decrease the distance between the two charges.
C decrease the mass of one of the objects
D increase the mass of one of the objects
Answers
Electric potential energy is the energy that is stored in a system of two or more electrically charged objects due to their position relative to each other. As the distance between the charged objects increases, the electric potential energy of the system decreases. This is because the electrical force between the objects decreases as the distance between them increases. Therefore, by increasing the distance between two positively charged objects, the electric potential energy of the system will decrease.
Decreasing the distance between the two charges (option B) would actually increase the electric potential energy of the system, as the electrical force between the objects would increase. Changing the mass of the objects (options C and D) would not have any effect on the electric potential energy of the system, as electric potential energy depends only on the charges and their separation distance.
At the boiling point of a liquid there is an increase in?
Heat of vaporisation
Potential energy
Free energy
Entropy which one is correct option
Answers
At the boiling point of a liquid, there is an increase in the heat of vaporisation. The correct option is Heat of vaporisation.What is boiling point?The boiling point of a liquid is the temperature at which the vapor pressure of a liquid is equal to the pressure above it, resulting in the formation of a vapor bubble within the liquid.
The boiling point of a substance is a characteristic physical property used to describe and identify it. Heat is absorbed during boiling because the temperature of the vapor is greater than that of the liquid.
How does heat of vaporisation relate to the boiling point?The heat of vaporisation, also known as the enthalpy of vaporisation, is the amount of energy required to turn a liquid into a gas at the boiling point, while the heat of fusion is the amount of energy required to melt a solid at its melting point. At the boiling point, a liquid's molecules begin to gain enough kinetic energy to escape the liquid phase and become a gas. As a result, the heat of vaporisation is the energy required to overcome intermolecular forces in a liquid to convert it into its gaseous form. Hence, at the boiling point of a liquid, there is an increase in the heat of vaporisation, which is the correct option.
To know more about boiling point of liquid visit:
brainly.com/question/32929883
#SPJ11
calculate the ph and pOH when pH is 2.37, 11.05, or 6.5
Answers
: When pH is 2.37, pOH can be calculated using the formula pH + pOH = 14. Therefore, pOH = 14 - 2.37 = 11.63.
Similarly, when the pH is 11.05, the pOH can be calculated as pOH = 14 - 11.05 = 2.95. Finally, when the pH is 6.5, the pOH can be calculated as pOH = 14 - 6.5 = 7.5.
Learn more about pOH at:
https://brainly.com/question/17144456
#SPJ1
Identify each of these substances as acidic, basic, or neutral.
___ pure water, pH = 7.0
___ lake water, pH = 6.5
___ baking soda solution, pH = 9
___ soapy water, pH = 12
Answers: neutral, acidic, basic, basic
Answers
Answer:pure water is neutral . Lake water is acidic. Baking soda and soapy water are basic.
Explanation: 7 is neutral. The higher on the ph scale like 1-6 is acidic. The lower on the scale 8-13 is base or alkaline.
Answer:
neutral
acidic
basic
basic
atom can
The ground state electronic configuration of
be represented by
Answers
Answer:
Ground state electronic configuration of nitrogen atom can be represented by 1, 4 only. All the unpaired electrons have spins aligned in the same direction only.
Explanation:
what is eukayotes cells and prokaryotes cells
Answers
Which of the following is a possible way to describe the H₂O component in the reaction below? 2HCl(aq) + Ca(OH)₂ (aq) → A. 2 molecules H₂O B. 1 molecule H₂O C. 2 LH₂O D. 4 moles H₂ 2H₂O(1) + CaCl₂(aq) 4
Answers
This equation shows that when hydrochloric acid (HCl) reacts with calcium hydroxide (Ca(OH)2), calcium chloride (CaCl2) and water (H2O) are produced.
Therefore, the H2O component in the reaction can be described as 2 molecules of water (2H2O), as shown in the balanced equation. Option A, "2 molecules H2O", is the correct way to describe the H2O component in the reaction.
2HCl(aq) + Ca(OH)₂ (aq) ⇒ A. 2 molecules H₂O B.
Option B, "1 molecule H2O", is incorrect as two molecules of water are produced in the reaction, not one.
Option C, "2 LH2O", is also incorrect as the symbol "L" is not used to represent water molecules in chemical equations.
Option D, "4 moles H2" is also incorrect as hydrogen gas (H2) is not produced in this reaction.
To know more about calcium hydroxide , visit:
https://brainly.com/question/9584549
#SPJ1
Think of an everyday situation in which two objects interact and exert a force on each other. Explain how the interaction can cause energy to be transferred from one object to the other
Answers
Answer:
It's explained below.
Explanation:
An everyday situation is when we raise an object.
Now, when we raise an object, energy is transferred to the Earth object system and thus the gravitational field energy of the system will increase.
Now, this energy is usually released when the object falls. The mechanism of this release is known as gravitational force.
In the same manner, two magnetic and electrically charged objects that are interacting at a distance will exert forces on each other and this can lead to transfer of energy between the interacting objects.
nR
ΔS
= (b) If instead, the pressure of the sample was maintained at 1.00 atm while it was cooled to −80.0
∘
C, what would be the entropy change as a fraction of nR ?
nR
ΔS
=
Answers
The entropy change as a fraction of nR is 0.
To calculate the entropy change (ΔS) as a fraction of nR when the pressure is maintained at 1.00 atm while cooling the sample to -80.0°C, we need to consider the ideal gas law and the relationship between entropy and temperature.
Step 1: Convert temperature to Kelvin
To use the ideal gas law and entropy formulas, we need to convert the temperature from Celsius to Kelvin.
T1 = -80.0°C + 273.15 = 193.15 K (initial temperature)
Step 2: Determine the final temperature
The final temperature is not given explicitly, but since the pressure is maintained constant, we can assume that the temperature changes to -80.0°C in this case as well.
T2 = -80.0°C + 273.15 = 193.15 K (final temperature)
Step 3: Calculate the entropy change
The entropy change (ΔS) for an ideal gas at constant pressure is given by the equation:
ΔS = nR ln(T2/T1)
Since the pressure is constant, the change in entropy is directly proportional to the change in temperature.
Step 4: Determine the fraction of nR
To express the entropy change as a fraction of nR, we divide the calculated ΔS by nR.
ΔS/nR = (nR ln(T2/T1)) / nR
ΔS/nR = ln(T2/T1)
Step 5: Calculate the entropy change as a fraction of nR
Plugging in the values for T1 and T2:
ΔS/nR = ln(193.15 K / 193.15 K)
ΔS/nR = ln(1)
ΔS/nR = 0
Therefore, the entropy change as a fraction of nR, when the pressure is maintained at 1.00 atm while cooling the sample to -80.0°C, is 0.
Learn more about entropy change from the given link: https://brainly.com/question/28244712
#SPJ11
calculate the molar concentration (m), molality (m), and % by mass (% m), for a solution formed by mixing 10.7 g of a solute, with a molar mass of 86 g/mol, with 155.7 g of solvent. (the density of the solution is 1.3 g/ml).
Answers
To calculate the molar concentration (m), we need to determine the number of moles of the solute and the volume of the solution.
First, let's calculate the number of moles of the solute:
Moles of solute = Mass of solute / Molar mass of solute
= 10.7 g / 86 g/mol
= 0.1244 mol
Next, let's calculate the volume of the solution:
Volume of solution = Mass of solvent / Density of solution
= 155.7 g / 1.3 g/ml
= 119.77 ml
Now, we can calculate the molar concentration (m):
Molar concentration (m) = Moles of solute / Volume of solution (in liters)
= 0.1244 mol / (119.77 ml / 1000 ml/L)= 1.038 MTo calculate the molality (m), we need to determine the mass of the solvent and the mass of the solute.
Mass of solvent = 155.7 gMass of solute = 10.7 gMolality (m) = Moles of solute / Mass of solvent (in kg)
= 0.1244 mol / (155.7 g / 1000 g/kg)= 0.7988 mTo calculate the percent by mass (% m), we need to determine the mass of the solute and the mass of the solution.
Mass of solute = 10.7 g
Mass of solution = Mass of solute + Mass of solvent
= 10.7 g + 155.7 g= 166.4 gPercent by mass (% m) = (Mass of solute / Mass of solution) * 100
= (10.7 g / 166.4 g) * 100= 6.43%Therefore, the molar concentration (m) is 1.038 M, the molality (m) is 0.7988 m, and the percent by mass (% m) is 6.43%.
Learn More About Moles at brainly.com/question/29367909
#SPJ11
PLEASE HURRY I NEED HELP, LIMITED TIME!!!!
Question- Convert 8.65 × 10^25 atoms H to its mass in grams
A. 5.26 x 10^49 g H
B. 1.45 g H
C. 1.01 g H
D. 145 g H
Answers
Answer:
Mass = 145g H
Explanation:
-First we need to convert atoms to moles :
1mole =6.022 ×10^23 atoms (The number of particles in 1 mole is called Avogadro's Number (6.0221421 x 1023))
8.65×10^23 atoms of H ×1 mol / 6.022×10^23
=1.45×10^2
=145 mol
- then convert moles to grams :
Mass in grams of H
Mass = number of moles ×molar mass for H
= 145 mol ×1 g/mol
= 145 g
An man in Arkansas recently found a 9 carat diamond at Crater of Diamonds State Park. Five carats are equivalent to one gram, so this diamond weighs 1.8 g. Diamond is a crystalline form of the element carbon. How many atoms of carbon are in this 1.8 g diamond
Answers
Answer:
The answer is "1.8 g diamond includes 9.03e22 atoms".
Explanation:
Given:
Weight of 9-carat diamond = 1.8 g
\(\therefore\\\\\)
weight of 1-mole carbon =12 g
\(\because\)
calculating the moles which are available into 18 g carbon weight:
x= Carbon moles weight in 1.8 g
\(1 - mol\ C = 12 g\\\\x - mol\ C = 1.8 g\\\\\to x = \frac{(1.8 \ g \times 1\ mol\ C)}{ 12 \ g}\\\\\to x = 0.15\ moles\)
In 1 mole element associated with Avogadro's number that is \(6.02 \times 10^{23}\\\\\)
Carbon includes in 1 mol\(=6.02 \times 10^{23}\ atoms\\\\\)
0.15 mol of carbon includes:
\(= 0.15 \times 6.02 \times 10^{23} = 9.03 \times 10^{22}\ atoms.\)
Using the scientific notation:
0.15 mol of carbon includes: 9.03e22 atoms. So, 1.8 g diamond includes 9.03e22 atoms.
what is the atomic number of the atom pictured?

Answers
The atomic number of the atom shown in the picture would be nine because there is a total of nine protons present inside the nucleus of the atom and the number of protons represents the atomic number of the atom.
The number of neutrons is unrelated to the atomic number of an atom.
What is the atomic number?An atom's atomic number is determined by the total number of protons it contains.
The number of neutrons is unrelated to the atomic number of an atom.
The atoms in the image would have an atomic number of nine since its nucleus contains a total of nine protons, and the number of protons corresponds to the atomic number of the atom.
Thus, the atomic number of the atom shown would be nine.
To learn more about the atomic number from here, refer to the link;
brainly.com/question/14190064
#SPJ2
What does “escape velocity” allow a rocket to do?
Answers
Answer:
With escape velocity in a direction pointing away from the ground of a massive body, the object will move away from the body, slowing forever and approaching, but never reaching, zero speed. Once escape velocity is achieved, no further impulse need be applied for it to continue in its escape.
Explanation:
Make sure to edit so you're not copy write
Answer: "With escape velocity in a direction pointing away from the ground of a massive body, the object will move away from the body, slowing forever and approaching, but never reaching, zero speed. Once escape velocity is achieved, no further impulse need be applied for it to continue in its escape".
Explanation: This might help lol
What affects wind speed?
Answers
Answer:
At the Earth's surface, wind blows horizontally from high pressure to low pressure areas. The speed is determined by the rate of air pressure change, or gradient, between the two pressure areas. The greater the pressure difference, the faster the winds.
- sciencing
The Four Forces That Influence Wind Speed & Wind Direction
Explanation:
The theory of continental drift, a theory of a single, massive landmass that may have broken apart into what we associate with the modern landmasses and major continents was first supported by what significant evidence?
Answers
Answer:
So its a theory by Alfred Wegener is that All land was one big piece of land called pangea in the early 20th century people began to think that land masses can move such as many do just from it being there or natural disasters then going in to more research they found that when putting the continents together it fit like a puzzle and the fact that some fossils were far away from were the same were found
The theory of continental drift, based their theory on several lines of evidence, including the fit of the continents, paleoclimate indicators, truncated geologic features, and fossils.
What does the continental drift theory?The continental drift hypothesis states that all the continents were once joined together as one large mass of land, but then the land spread apart and drifted into their current positions.
The hot magma flows in convection currents due to tremendous heat and pressure within the earth. These currents cause the tectonic plates that make up the earth's crust to move.
Thus, The theory of continental drift, based their theory on many lines of evidence, including the fit of the continents, paleoclimate indicators, truncated geologic features, and fossils.
To learn more about continental drift theory, follow the link;
https://brainly.com/question/28168801
#SPJ6
When the umbilical cord is tied after birth, the umbilical arteries close by filling in with. A) placental fluid. B) platelet plugs. C) connective tissue.
Answers
When the umbilical cord is tied after birth, the umbilical arteries close by filling in with connective tissue. The umbilical arteries carry deoxygenated blood from the fetus to the placenta, where the blood is oxygenated and returned to the fetus via the umbilical vein.
When the umbilical cord is cut, the flow of blood from the placenta to the fetus ceases, and the umbilical arteries and vein begin to constrict. This constriction is caused by the contraction of smooth muscles in the vessel walls and the closure of small valves within the vessels. As the umbilical arteries constrict, the flow of blood to the placenta decreases and the vessels begin to fill in with connective tissue. Over time, the connective tissue replaces the smooth muscle and valve tissue in the vessel walls, resulting in the complete closure of the umbilical arteries. This process is important to prevent bleeding and infection in the newborn.
Learn more about placenta here:
https://brainly.com/question/26959441
#SPJ11