A transition in the balmer series for hydrogen has an observed wavelength of 434 nm. Use the Rydberg equation below to find the energy level that the transition originated. Transitions in the Balmer series all terminate n=2.
Delta E= -2.178 x10-18J ( 1/n2Final - 1/n2Initial )
The number is 5.
What is the energy of this transition in units of kJ/mole? ( hint: the anser is NOT 4.58x10-22kJ/mole or -4.58x10-22kJ/mole)
Answers
Answer:
i. n = 5
ii. ΔE = 7.61 × \(10^{-46}\) KJ/mole
Explanation:
1. ΔE = (1/λ) = -2.178 × \(10^{-18}\)(\(\frac{1}{n^{2}_{final} }\) - \(\frac{1}{n^{2}_{initial} }\))
(1/434 × \(10^{-9}\)) = -2.178 × \(10^{-18}\) (\(\frac{n^{2}_{initial} - n^{2}_{final} }{n^{2}_{final} n^{2}_{initial} }\))
⇒ 434 × \(10^{-9}\) = (1/-2.178 × \(10^{-18}\))\(\frac{n^{2}_{final} *n^{2}_{initial} }{n^{2}_{initial} - n^{2}_{final} }\)
But, \(n_{final}\) = 2
434 × \(10^{-9}\) = (1/2.178 × \(10^{-18}\))\(\frac{2^{2} n^{2}_{initial} }{n^{2}_{initial} - 2^{2} }\)
434 × \(10^{-9}\) × 2.178 × \(10^{-18}\) = \((\frac{4n^{2}_{initial} }{n^{2}_{initial} - 4 })\)
⇒ \(n_{initial}\) = 5
Therefore, the initial energy level where transition occurred is from 5.
2. ΔE = hf
= (hc) ÷ λ
= (6.626 × 10−34 × 3.0 × \(10^{8}\) ) ÷ (434 × \(10^{-9}\))
= (1.9878 × \(10^{-25}\)) ÷ (434 × \(10^{-9}\))
= 4.58 × \(10^{-19}\) J
= 4.58 × \(10^{-22}\) KJ
But 1 mole = 6.02×\(10^{23}\), then;
energy in KJ/mole = (4.58 × \(10^{-22}\) KJ) ÷ (6.02×\(10^{23}\))
= 7.61 × \(10^{-46}\) KJ/mole
The initial energy level is 5 and the energy of this transition in units of kJ/mole is 7.57 * 10^-43 kJ/mole
We must first calculate ΔE as follows;
ΔE = hc/λ
h = Plank's constant = 6.6 * 10^-34 Js
c = speed of light = 3 * 10^8 m/s
λ = wavelength = 434 * 10^-9
ΔE = 6.6 * 10^-34 * 3 * 10^8/434 * 10^-9
ΔE = 0.0456 * 10^-17 J
ΔE = \(ΔE = -2.178 x10^-18 (\frac{1}{n^2final} - \frac{1}{n^2initial}) \\ΔE = -2.178 x10^-18 (\frac{1}{2^2} - \frac{1}{n^2initial} )\\\\4.56 * 10^-19/2.178 x10^-18 = (\frac{1}{2^2} - \frac{1}{n^2initial})\\0.210 = (\frac{1}{2^2} - \frac{1}{n^2initial})\\\frac{1}{n^2initial} = 0.25 - 0.210\\\frac{1}{n^2final} = 0.04\\n = (\sqrt{(0.04)^-1} \\n = 5\)
Energy of this transition in units of kJ/mole = 4.56 * 10^-19/ 6.02 * 10^23
= 7.57 * 10^-43 kJ/mole
Learn more; https://brainly.com/question/5295294
Related Questions
Select all statements that describe a general Diels-Alder reaction.
A. The reaction is initiated by heat.
B. The reaction involves the breaking of three π bonds.
C. The reaction is endothermic.
D. The reaction involves the formation of three new C-C σ bonds.
E. The reaction is concerted.
Answers
statements that describe Diels-Alder reaction. are A. The reaction is initiated by heat. D. The reaction involves the formation of three new C-C σ bonds. E. The reaction is concerted.
The Diels-Alder reaction is initiated by heat or light and involves the formation of new C-C sigma (σ) bonds. It is a concerted reaction, meaning that the breaking of the π bonds and the formation of the σ bonds occur simultaneously. The reaction is exothermic, not endothermic, as it releases energy upon the formation of the new bonds. Therefore, statements B and C are incorrect.
Learn more about Diels-Alder reaction here:
https://brainly.com/question/30474400
#SPJ4
An airplane flies due east from an airport.
How could the airplane provide evidence that the shape of Earth is a sphere?
Select the words from the drop-down menus to complete the explanation.
By continuing to fly east, and with enough fuel, the airplane will eventually reach the
. The longest eastward journey would occur from an airport
. Very short journeys would occur
Answers
The fact that an airplane flying due east from an airport will eventually return to its starting point indicates that the Earth is a sphere due to the curvature of the Earth's surface.
How can an airplane flying due east provide evidence for the shape of the Earth?
By continuing to fly east, and with enough fuel, the airplane will eventually reach the same airport it started from. The longest eastward journey would occur from an airport located at one of the Earth's poles. Very short journeys would occur near the equator.
The fact that the airplane returns to its starting point indicates that the Earth is a sphere, as it would not be possible on a flat plane. This phenomenon is due to the curvature of the Earth's surface, which causes the plane's trajectory to follow a circular path along the Earth's surface.
To learn more about airplane, visit: https://brainly.com/question/28502555
#SPJ1
The number of atoms in exactly 12 grams of carbon-12 is called
Answers
The number of atoms which are present exactly in 12 grams of carbon-12 is called Avogadro's number.
What is Avogadro's number?Avogadro's number is the number of atoms which are present in 1 mole of any substance and it is equal to 6.022×10²³.
And moles will be calculated as:
n = W/M, whereW = given massM = molar massMoles of Carbon-12 = 12g / 12g/mol = 1mole
In 1 mole of Carbon-12 = 6.022×10²³ atoms are present.
Hence, the number of atoms in exactly 12 grams of carbon-12 is called Avogadro's number.
To know more about Avogadro's number, visit the below link:
https://brainly.com/question/10614569
#SPJ2
How many grams of CH3OH must be added to water to prepare 275 mL of a solution that is 3.5 M CH3OH?
Answers
Answer:
30.8 grams
Explanation:
n = 3.5 mol/L × 0.275 L = 0.9625 mol
The molar mass of CH3OH is 32 g/mol.
Therefore, the mass of CH3OH required is:
mass = n × molar mass
mass = 0.9625 mol × 32 g/mol = 30.8 g
Therefore, you need 30.8 grams of CH3OH to prepare a solution that is 3.5 M CH3OH in 275 mL of water.
The vapor pressure of liquid hexane, C_6 H_14, is 40.0 mm Hg at 271 K. A sample of C_6 H_14 is placed in a closed, evacuated container of constant volume at a temperature of 400 K. It is found that all of the C_6 H_14 is in the vapor phase and that the pressure is 74.0 mm Hg. If the temperature in the container is reduced to 271 K, which of the following statements are correct? Choose all that apply. a) Liquid hexane will be present. b) Some of the vapor initially present will condense. c) Only hexane vapor will be present. d) No condensation will occur. e) The pressure in the container will be 50.1 mm Hg.
Answers
Answer:
Only Options (c), (d) and (e) are correct.
Explanation:
Without mincing words let's dive straight into the solution to the question above.
The following parameters from the question are given below which are going to aid in solving this question.
=> The vapor pressure of liquid hexane = 40.0 mmHg, Temperature, T1 = 271K.
=> The temperature, T2 = 400 K, the pressure,P2 = 74.0 mm Hg. The reduced temperature,T3 in the container = 271 K.
What is needed now is to Determine the final temperature, T3 = 271/400 × 74 = 50.01 mmHg.
Therefore, we only have the presence of the vapour of Hexane and there is no condensation. Hence, option c, d and e are the only correct options.
4. Long answer type questions: a. b. C. d. e. f. g. h. j. i. What are the constituent gases of air? Why is the surrounding air not seen with the eyes? How do you prove that air supports burning? How do you show that air occupies space? How do you prove that air has weight? How is air useful to us? Mention any three points. Write any three properties of air. How can you say that air exerts force? Write any four effects of air pollution. Write any three causes of air pollution and any two control measures of it.
Answers
1. The constituent gases of air are:
Nitrogen Oxygen Argon Carbon Dioxide2. The surrounding air is not seen with the eyes because it is transparent. Air molecules are not visible to the na-ked eye, and they do not scatter or absorb visible light significantly. Therefore, air appears colorless and transparent.
What is air?3. To prove that air supports burning, you can perform an experiment with a burning candle. Place a glass jar or bell jar over a lit candle, ensuring that the jar is airtight. As the candle burns, it consumes oxygen from the air inside the jar. Eventually, the candle flame will go out due to the lack of oxygen, proving that air (specifically oxygen) is necessary for burning.
4. To show that air occupies space, you can perform a simple experiment using a plastic bottle or syringe. Fill the bottle or syringe with water, ensuring there are no air bubbles. Then, cover the opening tightly and try to compress the air inside. You will find that it is not possible to compress the air significantly, indicating that air occupies space.
5. To prove that air has weight, you can use a sensitive balance or scale. Weigh an airtight container or balloon, and then fill it with air. The weight of the container or balloon with the added air will be greater than its initial weight, demonstrating that air has weight.
6. Air is useful to us in various ways. Three points highlighting the importance of air are:
Breathing and RespirationCombustion and Energy ProductionClimate Regulation7. Three properties of air include:
Air is Compressible: Air can be compressed or expanded under different conditions, allowing it to fill various spaces and containers.Air has Mass: Air molecules have mass, which means air itself has weight. It exerts pressure on objects and surfaces.Air Exerts Pressure: Due to the collisions of air molecules with surfaces, air exerts pressure in all directions. This pressure is known as atmospheric pressure.Air exerts force in various ways. For example, air pressure allows objects like airplanes to fly by providing lift. Air resistance or drag opposes the motion of objects moving through the air, creating a force that can affect their speed and trajectory.
8. Four effects of air pollution include:
Respiratory ProblemsEnvironmental Damage:Climate ChangeHuman Health Impacts9. Causes of pollution:
Industrial EmissionsVehicle EmissionsResidential and Agricultural Activities10. Two control measures for air pollution include:
Emission ReductionAir Quality RegulationsLearn more about air on https://brainly.com/question/15215203
#SPJ1
Helppppppppppppp plsssss

Answers
Answer:
Your weight would be greater.
If you travel to a high-gravity planet, your weight would be greater. Think of the astronauts' trip to the Moon — it's much smaller than Earth and has much less gravity, so the astronauts weighed less.
Your mass, however, cannot change.
A 3.69 g
sample of a compound consisting of carbon, hydrogen, oxygen, nitrogen, and sulfur was combusted in excess oxygen. This produced 2.08 g
CO2
and 1.28 g
H2O
. A second sample of this compound with a mass of 4.65 g
produced 4.77 g
SO3
. A third sample of this compound with a mass of 8.62 g
produced 3.48 g
HNO3
. Determine the empirical formula of the compound. Enter the correct subscripts on the given chemical formula.
Answers
The empirical formula of the compound is C₂H₁₆S₂N₃O.
What is the empirical formula of the compound?The moles of each element is as follows::
For CO₂:
Carbon (C) has a molar mass of 12.01 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Moles of C in CO₂ = 2.08 g / 12.01 g/mol = 0.173 moles
Moles of O in CO₂ = 2.08 g / 16.00 g/mol = 0.130 moles
For H₂O:
Hydrogen (H) has a molar mass of 1.01 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Moles of H in H₂O = 1.28 g / 1.01 g/mol = 1.27 moles
Moles of O in H₂O = 1.28 g / 16.00 g/mol = 0.080 moles
For SO₃:
Sulfur (S) has a molar mass of 32.06 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Moles of S in SO₃ = 4.77 g / 32.06 g/mol = 0.149 moles
Moles of O in SO₃ = 4.77 g / 16.00 g/mol = 0.298 moles
For HNO₃:
Hydrogen (H) has a molar mass of 1.01 g/mol.
Nitrogen (N) has a molar mass of 14.01 g/mol.
Oxygen (O) has a molar mass of 16.00 g/mol.
Moles of H in HNO₃ = 3.48 g / 1.01 g/mol = 3.45 moles
Moles of N in HNO₃ = 3.48 g / 14.01 g/mol = 0.248 moles
Moles of O in HNO₃ = 3.48 g / 16.00 g/mol = 0.217 moles
The simplest whole-number ratio of the elements will be:
Carbon: 0.173 moles / 0.080 moles ≈ 2.16
Hydrogen: 1.27 moles / 0.080 moles ≈ 15.88
Sulfur: 0.149 moles / 0.080 moles ≈ 1.86
Nitrogen: 0.248 moles / 0.080 moles ≈ 3.10
Oxygen: 0.080 moles / 0.080 moles = 1
Therefore, the empirical formula is C₂H₁₆S₂N₃O.
Learn more about empirical formulas at: https://brainly.com/question/1603500
#SPJ1
Congratulations you have worked hard and now you are done with the year! I am so proud of you!
Answers
Answer:
lololol
Explanation:
A 17.98-g piece of iron absorbs 2056.5 joules of heat energy, and its temperature changes from 25°C to 200°C. Calculate the specific heat capacity of iron.
Answers
Answer: \(0.65\ J/g.^{\circ}C\)
Explanation:
Given
Mass of iron piece is \(m=17.98\ g\)
Heat absorbed \(Q=2056.5\ J\)
Temperature changes from \(25^{\circ}C\ to\ 200^{\circ}C\) i.e.
\(\Delta T=200-25=175^{\circ}C\)
Heat absorbed is given by \(Q=mc\Delta T\quad [c=\text{specific heat of material}]\)
Insert the values
\(\Rightarrow 2056.5=17.98\times c\times 175\\\\\Rightarrow c=\dfrac{2056.5}{3146.5}\\\\\Rightarrow c=0.65\ J/g.^{\circ}C\)
Thus, the specific heat of iron is \(0.65\ J/g.^{\circ}C\)
Consider the following silica gel TLC plate of compounds A, B, and C developed in hexanes:
Consider the following silica gel TLC plate of com
a) Determine the R f values of compounds A, B, and C run on a silica gel TLC plate using hexanes as the solvent
b) Which compound, A, B, or C, is the most polar?
c) What would you expect to happen to the R f values if you used acetone instead of hexanes as the eluting solvent? (Think polarity of solvents)
Answers
The R f values for compounds A, B, and C on a silica gel TLC plate developed in hexanes would be determined by measuring the distance each compound traveled compared to the distance the solvent traveled.
a) There is a 4 cm gap between the origin and the solvent front. The Rf value for spot A is\(\frac{1.5}{4}= 0.375\), because it travelled 1.5 cm. Due to the 3.5 cm movement of Spot B, its Rf is\(\frac{3.5}{4} = 0.875\). Spot C shifted 3 cm, making its Rf \(\frac{3}{4} = 0.75\).
b)Due to its shorter travel distance than the other two compounds, compound A is the most polar. Recall that polar substances adhere to the adsorbent more readily, move less, and have a lower Rf value.
c)Hexanes is less polar than acetone as a solvent. Each of the three compounds would move more quickly if the same method were employed to elute them.The chemicals can be removed from the polar adsorbent more effectively with a more polar eluting solvent. Each compound would have a higher Rf value if acetone were used to elute the TLC plate as opposed to hexanes because each compound travels more quickly.
learn more about Rf value Refer:brainly.com/question/17796724
#SPJ1

PLEASE HELP!!!! WILL GIVE BRAINLIEST!!!

Answers
Answer:
im gonna say b
Explanation:
How many 1.5 M NaOH are require to neutralize 220 mL of 0.8 M HCl?
a. 144.8 mL
b 117.3 mL
c. 94.6 mL
d. 180. 1 mL
Answers
According to molar concentration , 117.3 ml of 1.5 M NaOH are required to neutralize 220 ml of 0.8 M HCl.
What is molar concentration?Molar concentration is defined as a measure by which concentration of chemical substances present in a solution are determined. It is defined in particular reference to solute concentration in a solution . Most commonly used unit for molar concentration is moles/liter.
The molar concentration depends on change in volume of the solution which is mainly due to thermal expansion. Molar concentration is calculated by the formula, molar concentration=mass/ molar mass ×1/volume of solution in liters.
In terms of moles, it's formula is given as molar concentration= number of moles /volume of solution in liters.
In the given example, molarity of NaOH is obtained by substituting given values in the formula, M₁V₁=M₂V₂,∴V₁=0.8×220/1.5=117.3 ml.
Thus , 117.3 ml of 1.5 M NaOH are required to neutralize 220 mL of 0.8 M HCl.
Learn more about molar concentration,here:
https://brainly.com/question/21841645
#SPJ1
Which of the following is NOT an advantage of a wireless network? convenience reliability security speed
Answers
Answer:
Security
Explanation:
A wireless network consists of good connection with fast speed, convenience, and reliability. Security is a major problem with wireless connection as someone can get your information by joining the same connection as you.
moles of each product that would form as a result of the decomposition of aspirin
Answers
The decomposition of aspirin (acetylsalicylic acid,\(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)).
The decomposition of aspirin (acetylsalicylic acid, \(C_{9} H_{8} O_{4}\)) can occur through the hydrolysis reaction, resulting in the formation of acetic acid (\(CH_{3} COOH\)) and salicylic acid (\(C_{7} H_{6}O_{3}\)). To determine the moles of each product formed, we need to consider the balanced chemical equation for the reaction:
\(C_{9} H_{8} O_{4} = > C_{7} H_{6}O_{3} +CH_{3} COOH\)
From the equation, we can see that for every 1 mole of aspirin, 1 mole of salicylic acid and 1 mole of acetic acid are produced.
Therefore, the moles of salicylic acid and acetic acid formed will be equal to the number of moles of aspirin that decomposes. If we know the amount of aspirin in moles, we can directly calculate the moles of each product based on stoichiometry.
For more question on aspirin
https://brainly.com/question/25794846
#SPJ8
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale). If this is true, what determines the difference between a slate and a gneiss rock that both are formed from shale? What role does the parent rock play in determining the type of metamorphic rock that will be formed?
Answers
According to the Foliated Metamorphic Rock Chart slate, phyllite, schist, and gneiss can all have the same parent rock (shale) is a true statement.
The parent rock, in this case shale, plays a significant role in determining the type of metamorphic rock that will be formed. The minerals and structure of the parent rock provide the starting material for the metamorphic rock, and the specific conditions under which the rock undergoes metamorphism determine the final characteristics of the metamorphic rock.What determines the difference between a slate and a gneiss rock that both are formed from shale?Slate, phyllite, schist, and gneiss are all types of metamorphic rocks that can be formed from shale, which is a sedimentary rock composed of clay and other fine-grained minerals. The specific type of metamorphic rock that is formed from shale depends on the conditions under which the shale undergoes metamorphism, including the temperature, pressure, and presence of fluids.
Slate is a fine-grained metamorphic rock with a uniform, flat surface and a layered structure. It is formed when shale undergoes low-grade metamorphism, which occurs at relatively low temperatures and pressures.
Therefore, Gneiss, on the other hand, is a medium- to coarse-grained metamorphic rock with a banded or wavy texture. It is formed when shale undergoes high-grade metamorphism, which occurs at higher temperatures and pressures.
Learn more about Metamorphic Rock from
https://brainly.com/question/1176274
#SPJ1
2.Pressure is a force, what causes this force
Answers
Answer:
Pressure is a stress. It is a scalar given by the magnitude of the force per unit area. In a gas, it is the force per unit area exerted by the change of momentum of the molecules impinging on the surface. ... It is this force, acting on the surface of the solid, that we call the force due to pressure.
Explanation:
I hope this helps
A Question 2 (2 points) Retake question
Potassium chlorate decomposes into potassium chloride and oxygen gas according to
the following equation:
2 KClO3 --> 2 KCl + 3 02
Use the equation above to answer the questions that follow:
How many moles of oxygen will be produced from the decomposition of 276 g of
potassium chlorate?
Do not put the answer in scientific notation. Do not include the substance as part of
your unit. Units should be all lowercase letters and singular (not plural) and no
spaces among units. You will choose from the following units:

Answers
Answer:
3.3765 Mol O2
Explanation:
There is no work for this problem
If I have 5 mole of a gas at a pressure of 7.6 atm and a volume of 11 liters, what is the temperature?
Answers
Answer:
203.75 K
Explanation:
use the ideal gas law equation: PV=nRT, R being the constant 0.08206 L-atm/mol-K.
plug in the values given:
(7.6 atm) (11.0 L) = (5.0 mol) (0.08206 L-atm/mol-K) (T)
and solve for T!
The number that represents a neutral pH is . (2 points)
Answers
Answer:
7
Explanation:
Answer:
7 is your answer
YW! :)
Explanation:
As a scuba diver descends toward the bottom of the ocean, she experiences the sensation of her ears "popping." Based on your observations from the Can Crush Lab, why would this happen? (2 points)
Decreased pressure inside the diver's oxygen tank causes pressure on the diver's ears.
Increased pressure inside the diver's oxygen tank causes pressure on the diver's ears.
Pressure increases on the diver as the diver descends deeper underwater.
Pressure decreases on the diver as the diver descends deeper underwater
Answers
Answer: Pressure increases on the diver as the diver descends deeper underwater.
Explanation:
I need help!!!!!!!!!!!!!!!!
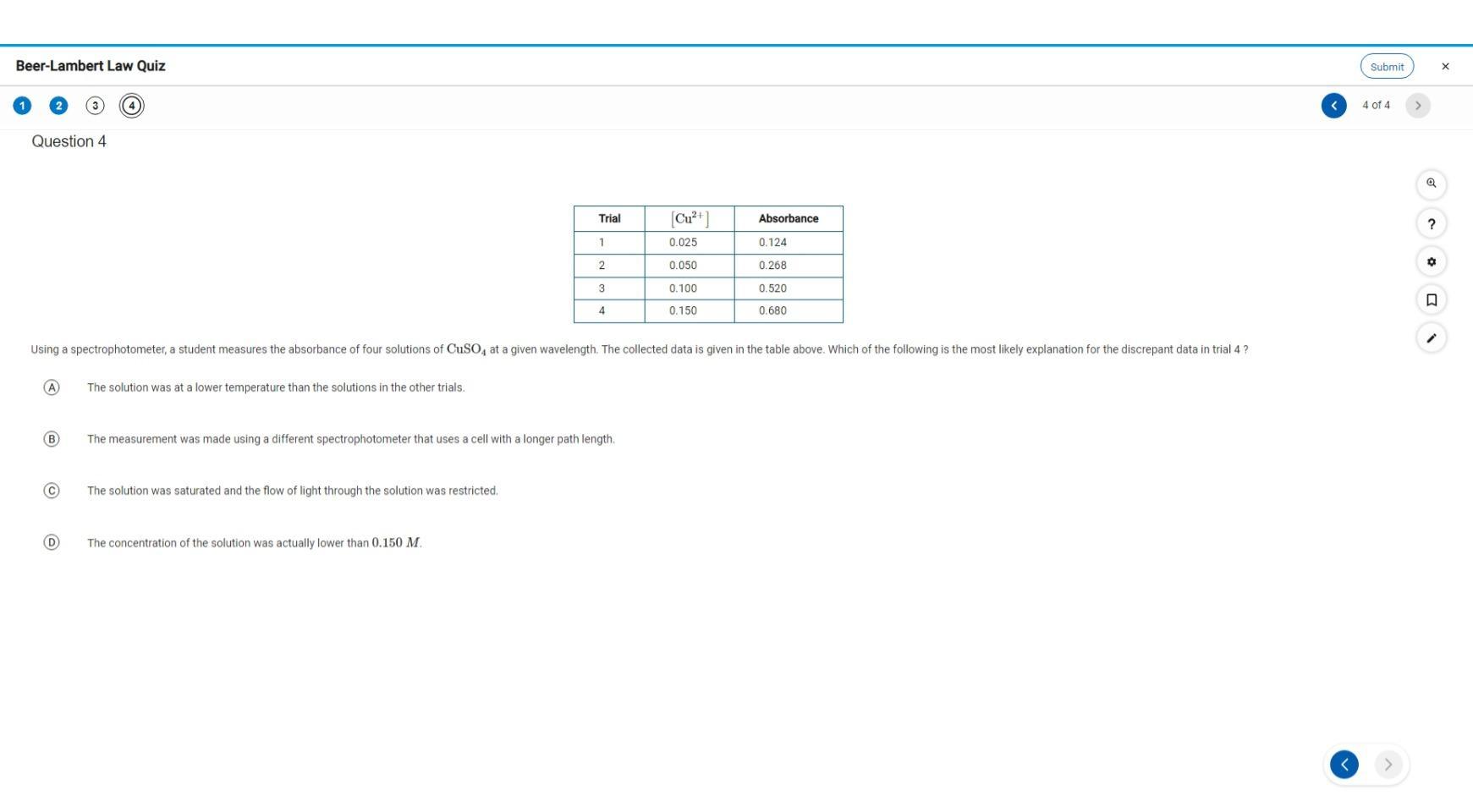
Answers
The reason for the fourth measurement is that the solution is saturated. Option C in the question.
What is the Beer Lambert's law?According to the Beer Lambert's law, the absorbance of the solution is found to the proportional to the concentration of the solution. This implies that as the concentration of the solution increases, the absorbance of the solution also increases.
The absorbance is proportional to the concentration, and the pathlength of the cell where the measurement is taken.
We can now see from the table that the absorbance of the solution is largely affected by the concentration of the solution and not really the pathlength.
Learn more about Beer Lambert's law:https://brainly.com/question/8831959
#SPJ1
Producer gas has the following composition by volume: O2 . The oxygen fed from the air and the producer gas is 20% in excess of 68.0 1.2507 2CO + O2 2CO2 The oxygen is from the air whose volumetric composition is assumed to be 80% N2 % Density, kg/m3 The gas is burned with oxygen according to the following equation: CO2 3.5 1.9768 the amount required for complete combustion. The combustion is 98% complete. N2 CO 28.0 1.2501 0.5 1.4289 and 20% O2 Carry out total material balance for this process based on 100kg of gas burned.
Answers
Answer:
ANSWER: (a) 6.93 m3 (b) 54.286 m3 (c) 20.45% CO2, 1.3496 02, 78.21% N2
Explanation:
mark me as a brain list
PLS HELP ASAP I NEED NUMBER 8

Answers
Explanation:
determine the genotypes of the parent organisms.
write down your "cross" (mating)
draw a p-square.
4. " ...
determine the possible genotypes of the offspring by filling in the p-square.
summarize results (genotypes & phenotypes of offspring)
How many water molecules are contained in a 0.50L bottle of water? Assume water has a density of 1.00 g/mL.
Answers
18.4 × 10^(24) water molecules are contained in a 0.50L bottle of water.
Given,
Density of water = 1.00 g/mL
Volume of water = 0.50L = 500 ml
We can calculate the mass of water.
As we know that,
Density = mass/ volume
By substituting the values, we get
1 = Mass / 500
Mass = 500 g.
Now, we know
Molar mass of water = 18 g
Mass of water = 500g
We will calculate the moles of water
Mole = given mass / molar mass
= 500/18 = 27.7 moles
As we know that,
1 mole = 6.673 × 10^(23) molecules
So, 27.7 moles contain = 6.673 × 27.7 × 10^(23)
= 18.4 × 10^(24) molecules
Thus, we concluded that 18.4 × 10^(24) water molecules are contained in a 0.50L bottle of water.
learn more about Density:
https://brainly.com/question/952755
#SPJ13
DNA is made up of repeating sequences of?
Answers
Answer:
Nucleic Acids
Explanation:
DNA, Deoxyribonucleic acid, is made up up nucleic acids. These nucleic acids are:
Adenine
Guanine
Cytosine
Thymine
Adenine binds to Thymine and Cytosine binds to Guanine. They are then "twisted" into a double helix.
Answer:
Explanation:
from two to several thousand base pairs and is estimated to constitute about 30% of the genome. Many of these sequences are localized in centromeres and telomeres, but they are also dispersed throughout the genome.
Calculate the cost of one pound of nitrogen from a fertilizer containing 30.0 % sodium nitrate by weight and costing $9.00 per 100 lb. Express your answer in dollars per pound of nitrogen to three significant figures.
Answers
Answer:
1.82 lb
Explanation:
Given that:
The chemical formula for sodium nitrate is NaNO₃, thus its molar mass = 84.99 g/mole
Also, the atomic mass of Nitrogen (N) = 14 g/mole
Thus; the mass of N in NaNO₃ = (14/84.99) × 100
the mass of N in NaNaNO₃ = 16.473 %
The cost of the fertilizer = $9.00/100 lb
Hence, for one pound of Nitrogen(N) from the fertilizer containing NaNO₃; we have:
= 100/16.473 lb of NaNO₃
= 6.0705 lb of NaNO₃
Given that the fertilizer contains 30% of NaNO₃
The needed amount of fertilizer = 6.0705 × (100/30)
The needed amount of fertilizer =20.235 lb
Thus the cost of one pound of nitrogen from the fertilizer is:
= ( $9.00/100 lb ) × 20.235 lb
= 1.82 lb
To calculate the cost of one pound of nitrogen from this fertilizer is $1.82
Given the following data:
Percent by mass of \(NaNo_3\) = 30%Cost of \(NaNo_3\) = $9.00 per 100 lb.Note: Sodium nitrate = \(NaNo_3\)
Molar mass of \(NaNo_3\) = 84.99 g/mol.
Atomic mass of nitrogen (N) = 14.00 g/mol.
To calculate the cost of one pound of nitrogen from this fertilizer:
First of all, we would determine the mass of nitrogen contained in sodium nitrate.
\(Mass \;of \;N = \frac{14}{84.99} \times 100\\\\Mass \;of \;N = \frac{1400}{84.99}\)
Mass of N = 16.47 grams.
Next, we would find a pound of nitrogen in the fertilizer:
\(One\; pound \;of\;N = \frac{100}{16.47}\)
One pound of nitrogen = 6.07 lb of \(NaNo_3\)
Also, we would calculate the amount of nitrogen in \(NaNo_3\) required from the fertilizer.
\(Amount \;of\;N = \frac{100}{30} \times 6.07\\\\Amount \;of\;N = \frac{607}{30} \\\\Amount \;of\;N = 20.2\;lb\)
Now, we can calculate the cost of one pound of nitrogen from this fertilizer:
\(Cost = \frac{9.00}{100} \times 20.2\\\\Cost = \frac{181.8}{100} \\\\Cost = 1.818\)
To three significant figures:
Cost = $1.82
Read more: https://brainly.com/question/16906167
suppose you start with gly fixed to the substrate. call this r1-coo- where the r1 stands for the solid substrate and all of gly except the coboxylic acid group. draw all of the resultant molecules when ala is allowed to react with the r1-coo-. be sure to include any charged atoms, assuming ph
Answers
When alanine (Ala) reacts with the substrate (r1-COO-), a peptide bond is formed between the carboxyl group of the substrate (COO-) and the amino group of the alanine (-NH3+).
This results in the formation of the molecule r1-Ala-COO-, where the "r1" indicates the solid substrate and "Ala" represents the alanine. At a neutral pH, the carboxyl group of the substrate will have a negative charge (COO-), while the amino group of alanine will have a positive charge (-NH3+). The formation of the peptide bond results in a molecule with a neutral charge.
It is important to note that the carboxyl group of the substrate and the amino group of alanine can react with other substrate or amino acid molecules to form additional peptide bonds, leading to the formation of longer peptide chains. This reaction is the basis of peptide synthesis and is used in the production of peptides, proteins, and other biologics.
Learn more about alanine:
brainly.com/question/19088437
#SPJ4

How many ways can you recall to synthesize
Answers
there are an infinite number of ways to synthesize an answer to a question, including the following:
Summarize the key points in a concise manner.
Provide a detailed explanation of the topic.
Use examples or analogies to illustrate the concept.
Break down the answer into smaller, more digestible pieces.
Address potential counterarguments or alternative perspectives.
Incorporate relevant statistics or data to support the answer.
Compare and contrast different aspects of the topic.
Provide historical context or a timeline of events.
Use a storytelling approach to engage the reader.
Use a Q&A format to organize the information.
To know more about answer synthesize, visit:
https://brainly.com/question/30029537
#SPJ1
1)Grignard reagent when reacted with methanol will yield A) ethanol (B) secondary alcohols (C) tertiary alcohols (D ropanol (E) primary alcohol
Answers
When the reaction of Grignard reagent reacted with methanol will yield a tertiary alcohol. Therefore, Option C tertiary alcohol is correct.
Contains a carbon-metal link, Grignard reagents are chemicals used in catalysis. They generally result from the anhydrous reaction of magnesium metal with an alkyl or aryl halide. Because of their high reactivity, Grignard reagents frequently act as nucleophiles in organic reactions.
An alkyl group from a Grignard reagent binds to the oxygen atom of methanol (CH3OH) when it interacts with the methanol, breaking the carbon-metal connection. A precursor alkoxide is created as a result. The equivalent alcohol is then produced by protonating the intermediate alkoxide.
The reaction of a Grignard reagent with methanol leads to the formation of a tertiary alcohol.
Learn more about reagents here:
https://brainly.com/question/29729676