A student dissolves 11.0 g of ammonium nitrate (NH4NO2) in 250. g of water in a well-insulated open cup. She then observes the temperature fall from 22.0°C to 18.5 °C over the course of 7.2 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NH, NO3(s) + NH(aq) + NO3(aq) You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to 2 significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. exothermic Is this reaction exothermic, endothermic, or neither? endothermic xs ? O neither If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. ДkJ Calculate the reaction enthalpy AH, xn per mole of NH,NOZ.
Answers
The reaction enthalpy (ΔH) per mole of NH₄NO₂ is approximately 26.7 kJ/mol.
The given reaction, NH₄NO₂(s) → NH₄⁺(aq) + NO₂⁻(aq), is endothermic. To calculate the amount of heat absorbed or released by the reaction, we can use the equation:
q = mcΔT
where q is the heat absorbed or released, m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature.
Mass of water (m) = 250. g
Change in temperature (ΔT) = 22.0°C - 18.5°C = 3.5°C
The specific heat capacity of water is 4.18 J/g·°C.
Converting the mass of water to grams: m = 250. g
Calculating the heat (q):
q = (250. g)(4.18 J/g·°C)(3.5°C)
q = 3662.5 J
Converting joules to kilojoules:
q = 3662.5 J / 1000 = 3.66 kJ
The reaction enthalpy (ΔH) per mole of NH₄NO₂ can be calculated by dividing the heat by the number of moles of NH₄NO₂ used.
First, we need to calculate the number of moles of NH₄NO₂:
Mass of NH₄NO₂ = 11.0 g
Molar mass of NH₄NO₂ = 80.04 g/mol
Number of moles (n) = mass / molar mass
n = 11.0 g / 80.04 g/mol
n ≈ 0.137 moles
Now we can calculate the reaction enthalpy:
ΔH = q / n
ΔH = 3.66 kJ / 0.137 moles
ΔH ≈ 26.7 kJ/mol
Learn more about enthalpy at https://brainly.com/question/29888663
#SPJ11
Related Questions
.Pb2+
Write electron configurations for the following ions.
Part A:
Ru3+
Express your answer in condensed form in order of increasing orbital energy as a string without blank space between orbitals. For example, [He]2s22p2 should be entered as [He]2s^22p^2.
Part B
As3−
Express your answer in condensed form in order of increasing orbital energy as a string without blank space between orbitals. For example, [He]2s22p2 should be entered as [He]2s^22p^2.
Part C
Y3+
Express your answer in condensed form in order of increasing orbital energy as a string without blank space between orbitals. For example, [He]2s22p2 should be entered as [He]2s^22p^2.
Part D
Pd2+
Express your answer in condensed form in order of increasing orbital energy as a string without blank space between orbitals. For example, [He]2s22p2 should be entered as [He]2s^22p^2
Answers
Part A: Ru3+ The electron configuration for Ru is [Kr]5s^24d^6. To form Ru3+, three electrons are removed. Therefore, the electron configuration for Ru3+ is [Kr]4d^5.
Part B: As3-
The electron configuration for As is [Ar]4s^23d^104p^3. To form As3-, three electrons are gained. Therefore, the electron configuration for As3- is [Ar]4s^23d^104p^6.
Part C: Y3+
The electron configuration for Y is [Kr]5s^24d^15p^1. To form Y3+, three electrons are removed. Therefore, the electron configuration for Y3+ is [Kr]4d^0.
Part D: Pd2+
The electron configuration for Pd is [Kr]5s^04d^10. To form Pd2+, two electrons are removed. Therefore, the electron configuration for Pd2+ is [Kr]4d^8.
Part A: Ru3+
The electron configuration for Ru is [Kr]5s^24d^6. To form Ru3+, three electrons are removed. This means that the 5s orbital is emptied and one electron is removed from the 4d orbital. Therefore, the electron configuration for Ru3+ is [Kr]4d^5. This configuration indicates that there are 5 electrons in the 4d orbital.
Part B: As3-
The electron configuration for As is [Ar]4s^23d^104p^3. To form As3-, three electrons are gained, filling up the 4p orbital. Therefore, the electron configuration for As3- is [Ar]4s^23d^104p^6. This configuration indicates that there are 6 electrons in the 4p orbital.
Part C: Y3+
The electron configuration for Y is [Kr]5s^24d^15p^1. To form Y3+, three electrons are removed. This means that the 5s and 4d orbitals are emptied. Therefore, the electron configuration for Y3+ is [Kr]4d^0. This configuration indicates that there are no electrons in the 4d orbital.
Part D: Pd2+
The electron configuration for Pd is [Kr]5s^04d^10. To form Pd2+, two electrons are removed, leaving the 5s and 4d orbitals empty. Therefore, the electron configuration for Pd2+ is [Kr]4d^8. This configuration indicates that there are 8 electrons in the 4d orbital.
Learn more about electron configurations here: brainly.com/question/29157546
#SPJ11
what happens to the oxidation number of one of its elements when a compound is oxidized?
Answers
When a compound is oxidized, one of its elements may experience a change in oxidation number.
Oxidation is the loss of electrons, so if an element loses electrons, its oxidation number increases. For example, in the compound H2O, the oxidation number of oxygen is -2 and the oxidation number of hydrogen is +1. If H2O is oxidized, the oxygen may gain electrons and its oxidation number will decrease.
At the same time, the hydrogen may lose electrons and its oxidation number will increase. This change in oxidation numbers is important in understanding chemical reactions and the transfer of electrons between molecules.
More on oxidation: https://brainly.com/question/13182308
#SPJ11
HELP ME I HAVE THIS REALLY IMPORTANT TEST THAT I NEED TO DO FAST!!|
According to this pie graph, the majority of fresh water in the world is used for household purposes. true or false
Answers
Answer:
TRUE!
Explanation:
in the ostwald process, 4 molecules of ammonia and 5 molecules of oxygen react to produce 4 molecules of nitric oxide and 6 molecules of water. which
Answers
In the Ostwald process, 4 molecules of ammonia and 5 molecules of oxygen react to produce 4 molecules of nitric oxide and 6 molecules of water.
What is the role of the platinum-rhodium gauze catalyst in the Ostwald process?The platinum-rhodium gauze catalyst is used in the first step of the Ostwald process to catalyze the oxidation of ammonia with oxygen to produce nitric oxide and water. The catalyst helps to lower the activation energy of the reaction and increase the rate of reaction.
The Ostwald process is a chemical process used to produce nitric acid (HNO3) from ammonia (NH3) and oxygen (O2). The process involves two main steps. In the first step, ammonia is oxidized with oxygen to produce nitric oxide (NO) and water (H2O) according to the following reaction:
4NH3 + 5O2 → 4NO + 6H2O
This reaction is exothermic and is typically carried out in a catalytic reactor at temperatures between 800°C and 900°C using a platinum-rhodium gauze catalyst.
In the second step, the nitric oxide is further oxidized with air to produce nitrogen dioxide (NO2) which is then absorbed in water to produce nitric acid:
2NO + O2 → 2NO2
3NO2 + H2O → 2HNO3 + NO
The nitrogen dioxide (NO2) is typically produced by passing the nitric oxide (NO) through a bed of vanadium oxide catalysts and then mixing it with air.
The Ostwald process is an important industrial process for the production of nitric acid, which is used in the production of fertilizers, dyes, and other chemicals.
Learn more about Ostwald process here:
https://brainly.com/question/28295415
#SPJ1
Question 14 PM2.5 is defined as ________
- the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air equal to 2.5 micrometers in diameter. - the mass concentration of particles in the air greater than or equal to 2.5 micrometers in diameter. Question 15 Carbon dioxide (CO2) is a criteria air pollutant. - True - False Question 16 Roughly percent of emissions of carbon monoxide in Santa Clara County come from mobile sources (select the choice closest to the correct answer). - 50 - 75 - 25 Question 17
The term "photochemical smog" is most synonymous with which of the following criteria air pollutants? - lead (Pb) - carbon monoxide (CO) - sulfur dioxide ( SO2) - ozone (O3) Question 18 "Attainment" of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards. - True - False
Answers
: PM2.5 is defined as the mass concentration of particles in the air less than or equal to 2.5 micrometers in diameter.Question 15: False, carbon dioxide (CO2) is not considered a criteria air pollutant.
Question 16: The closest answer is 50%, but the exact percentage is not provided in the question.Question 17: The term "photochemical smog" is most synonymous with ozone (O3), which is a criteria air pollutant.Question 18: True, attainment of ambient air quality standards requires that measured concentrations at all monitoring stations within an air district are below ambient air standards.
Question 14 asks about the definition of PM2.5. PM2.5 refers to particulate matter with a diameter less than or equal to 2.5 micrometers. It represents the mass concentration of particles suspended in the air, which are small enough to be inhaled into the respiratory system and can have adverse health effects.
Question 15 states whether carbon dioxide (CO2) is a criteria air pollutant. Criteria air pollutants are a set of pollutants regulated by environmental agencies due to their detrimental impact on air quality and human health. However, carbon dioxide is not considered a criteria air pollutant because it does not directly cause harm to human health or the environment in the same way as pollutants like ozone or particulate matter.
Question 16 asks about the percentage of carbon monoxide (CO) emissions from mobile sources in Santa Clara County. While the exact percentage is not provided in the question, the closest answer option is 50%. However, it is important to note that the precise percentage may vary depending on specific local conditions and emissions sources.
Question 17 inquires about the criteria air pollutant most synonymous with the term "photochemical smog." Photochemical smog is primarily associated with high levels of ground-level ozone (O3). Ozone is formed when nitrogen oxides (NOx) and volatile organic compounds (VOCs) react in the presence of sunlight, creating a hazy and polluted atmospheric condition.
Question 18 addresses the concept of "attainment" of ambient air quality standards. To achieve attainment, measured concentrations of pollutants at all monitoring stations within an air district must be below the established ambient air quality standards. This ensures that the air quality in the given area meets the required standards for protecting human health and the environment.
Learn more about mass concentration here:- brainly.com/question/23437000
#SPJ11
what is the mass of sodium chloride required to create a 0.875 m solution 534 g of water. how many moles of nacl is required
Answers
The mass of sodium chloride that is required to create a 0.875 M solution 534 g of water is 27.291 g and 0.467 moles of NaCl is required.
Mass of water = 534 g
Molality of the solution = 0.875 m
Molality is the number of moles of solute per kilogram of solvent.
It is represented by the formula:
Molality = number of moles of solute / kilogram solvent
Its mathematical expression is:
m = n/kg
Now we will convert the g into kg.
Mass of water = 534 g× 1kg/1000 g = 0.534 kg
putting the values in formula:
0.875 m = n / 0.534 kg
n = 0.467 mol
Now we will calculate the mass of sodium chloride:
Mass = number of moles × molar mass
Mass = 0.467 mol × 58.44 g/mol
Mass = 27.291 g
Thus, the required mass and moles of NaCl are 27.291g and 0.467mol respectively.
To know more about molality here
https://brainly.com/question/27987133
#SPJ4
you have 0.5 l of air at 203 k in an expandable container at constant pressure. you heat the container to 273 k. what is the volume of air? responses 0.25 l 0.25 l 0.37 l 0.37 l 0.67 l 0.67 l 1.5 l
Answers
The volume of air at 273 K would be 0.67 L by Gas Law. The ideal gas law, a state equation for all gases, states: PV is equal to nRT, where n is the number of moles of the gas and 8.314 joules per kelvin per mole is the universal (or perfect) gas constant.
According to the Ideal Gas Law, all gases have an equal number of molecules when they are at the same temperature, pressure, and volume (but not the same mass)
V1=0.5 L
T1=203 K
T2 = 273 K
V2 = unknown.
Now, \(\frac{V1}{T1}\) = \(\frac{V2}{T2}\)
So,
0.5L/203 = V2/273
V2= 0.67 L
So, Volume of gas will be 0.67 L.
Learn more about Gas Law
brainly.com/question/13207402
#SPJ4
how many carbon dioxide molecules are produced if 8.45 x 1023 of water molecules of water are produced
Answers
The given question is incomplete. The complete question is:
Determine how many carbon dioxide molecules are produced if molecules of water are produced
\(C_2H_6(g)+O_2 (g)\rightarrow CO2(g)+H_2O(g)\)
Answer: \(5.60\times 10^{23}\) molecules of carbon dioxide are produced
Explanation:
The balanced chemical equation is:
\(2C_2H_6(g)+7O_2 (g)\rightarrow 4CO2(g)+6H_2O(g)\)
\(\text{Moles of water}=\frac{\text{given molecules}}{\text{Avogadros number}}=\frac{8.45\times 10^{23}}{6.023\\times 10^{23}}=1.40moles\)
Accoding to stoichiometry:
6 moles of water are produced along with = 4 moles of carbon dioxide
Thus 1.40 moles of water are produced along with = \(\frac{4}{6}\times 1.40=0.93\) moles of carbon dioxide
Molecules of carbon dioxide = \(moles\times {\text {Avogadros number}}=0.93\times 6.023\times 10^{23}=5.60\times 10^{23}\)
What might happen if water molecules did not have a slight negative charge on one end and a slight positive charge on the other? Polar molecule
Answers
If water molecules did not have a slight negative charge on one end and a slight positive charge on the other then Water would be incapable of freezing or boiling.
What is water?Water is an inorganic polar chemical. At room temperature, it is an odorless and tasteless liquid with a tinge of blue. This most basic hydrogen chalcogenide is unquestionably the most researched chemical compound and therefore is known as that of the "universal solvent" due to its propensity to dissolve a wide range of compounds.
If water molecules did not have a slight negative charge on one end and a slight positive charge on the other then Water would be incapable of freezing or boiling.
Therefore, water would be incapable of freezing or boiling.
To learn more about water, here:
https://brainly.com/question/16630851
#SPJ9
What two of the following organisms are secondary consumers in this food web?
Answers
Secondary consumers are organisms that primarily feed on herbivores or other primary consumers.
They occupy the next trophic level above the primary consumers in a food web. They obtain energy by consuming the primary consumers and play an important role in regulating the population of herbivores.
Examples of commonly observed secondary consumers include:
Carnivorous mammals: Animals such as wolves, lions, and tigers that feed on herbivores like deer, zebras, or gazelles.
Birds of prey: Species like eagles, hawks, and owls that consume small mammals, reptiles, or other birds.
Carnivorous fish: Fish like pike, barracuda, or bass that prey on smaller fish or aquatic invertebrates.
Predatory insects: Insects such as spiders, mantises, or dragonflies that feed on other insects, including herbivorous insects.
In a specific food web, the identification of secondary consumers would depend on the specific organisms present and their feeding interactions. It would be necessary to analyze the trophic relationships among the organisms in the food web to determine the secondary consumers accurately.
For more such questions on Secondary consumers visit:
https://brainly.com/question/28631974
#SPJ8
why does the less reactive ion participate in electrolysis, instead of the higher reactive metal??
Answers
Answer:
In a reactivity series, the most reactive element is placed at the top and the least reactive element at the bottom. More reactive metals have a greater tendency to lose electrons and form positive ions .
Explanation:
Yes ur well come
Using standard heats of formation, calculate the standard enthalpy change for the following reaction. 2CO(g) + 2NO(g)2CO2(g) + N2(g)
Answers
The standard enthalpy change for the given reaction is -1266.2 kJ/mol. This indicates that the reaction is exothermic, and that the formation of the products releases energy to the surroundings.
The standard enthalpy change for the given reaction can be calculated using the standard heats of formation (∆Hf) of the reactants and products. The standard heat of formation (∆Hf) is the enthalpy change that occurs when one mole of a substance is formed from its elements in their standard states at a specified temperature and pressure.The balanced chemical equation for the reaction is:2CO(g) + 2NO(g) → 2CO2(g) + N2(g)The standard heats of formation (∆Hf) for the reactants and products are:\(∆Hf(CO(g)) = -110.5 kJ/mol\)\(∆Hf(NO(g)) = 90.4 kJ/mol\)\(∆Hf(CO2(g)) = -393.5 kJ/mol\)\(∆Hf(N2(g)) = 0 kJ/mol\)The standard enthalpy change (∆H°) for the given reaction can be calculated using the formula:\(∆H° = ∑n∆Hf(products) - ∑n∆Hf(reactants)\)where n is the stoichiometric coefficient of each species in the balanced equation.Substituting the values of standard heats of formation into the formula, we get:\(∆H° = [2(-393.5 kJ/mol) + 1(0 kJ/mol)] - [2(-110.5 kJ/mol) + 2(90.4 kJ/mol)]\)= -1266.2 kJ/molTherefore, the standard enthalpy change for the given reaction is -1266.2 kJ/mol. This indicates that the reaction is exothermic, and that the formation of the products releases energy to the surroundings.For more such question on standard enthalpy
https://brainly.com/question/30431725
#SPJ11
What type of central-atom orbital hybridization corresponds to each electron-group arrangement:
(b) octahedral;
Answers
the type of central-atom orbital hybridization corresponds to each electron-group arrangement
octahedral
What is Octahedral molecular geometry?
Octahedral molecular geometry describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron.The bond angle is 900.Example: SF6, TeF6 etc.To learn more about Octahedral molecular geometry
brainly.com/question/14007686
#SPJ4
strength of acids how does the molecular structure of an acid influence its strength
Answers
The strength of acids is influenced by the molecular structure of an acid by polarization of H-A bond and electronic effects like inductive, resonance .
The more the acidic strength, the more is the ability of an acid to donate H+ ions. So, the molecular structure of an acid is very important in determining the strength of the acid. Generally, if the size of an acid molecule increases, the acidic strength increase. If the size of the acid molecule decreases, the acidic strength decreases. There might be few exceptions. In conclusion, the strength of acids is greatly influenced by the molecular structure of an acid.
The following factors affect the acidic strength of acids :
Polarization of the H-A bond: The more polarized the H-A bond, the stronger the acid. This is because a polarized bond means that the electrons are more strongly attracted to one atom (A) than the other (H). This makes it easier for the H atom to be released as a proton (H+). Inductive effect: The inductive effect is a type of electron delocalization that can occur in molecules with multiple atoms. It occurs when electrons are pulled towards atoms that are more electronegative. Inductive effects can weaken the H-A bond, making the acid stronger. Resonance: Resonance is a phenomenon that occurs when a molecule can be represented by multiple Lewis structures that have the same overall electron configuration. Resonance can stabilize a molecule by delocalizing electrons. In the case of acids, resonance can stabilize the conjugate base, making the acid stronger.In general, the key factors that determine the strength of an acid are the presence of polar bonds, the stability of the resulting conjugate base, and the ability to release hydrogen ions (protons).
Thus, the strength of acids is influenced by the molecular structure of an acid by polarization of H-A bond and electronic effects like inductive, resonance .
To learn more about acids :
https://brainly.com/question/15516010
#SPJ11
Reaction between silver nitrate and sodium chloride. A balanced chemical equation
Answers
Answer:
NaCl(aq) + AgNO3(aq) Sodium Silver chloride nitrate AgCl(s) + NaNO3(aq) Silver Sodium chloride nitrate
Propane, the gas used in barbeque grills, is made of
carbon and hydrogen. Will the atoms that make up
propane form covalent bonds? Why or why not?
Answers
Explanation: When two or more non-metals bond together, they always bond covalently to fill their last electron shell and gain stability.
Answer:
The atoms of propane will form covalent bonds because carbon and hydrogen are both nonmetals.
Explanation:
sample response
A metal salt with the formula MCl2 crystallizes from water to form a solid with the composition MCl2⋅6H2O. The equilibrium vapor pressure of water above this solid at 298 K is 19.9 mbar
Part A
What is the value ofΔrG for the reaction
MCl2⋅6H2O(s)⇌MCl2(s)+6H2O(g)
when the pressure of water vapour is 19.9 mbar ?
Express your answer as an integer with the appropriate units.
part B
What is the value of ΔrG ∘∘ when the pressure of water vapour is 1 bar?
Express your answer with the appropriate units.
Answers
The value of ΔrG is approximately 190.4 kJ/mol when the pressure of water vapor is 19.9 mbar, the value of ΔrG is -57.8 kJ/mol when the pressure of water vapor is 1 bar.
We can use the relationship between ΔG and equilibrium constant Kp to find ΔrG:
ΔrG = -RT ln(Kp)
First, we need to find Kp. The pressure of water vapor above the solid is given as 19.9 mbar, which is equivalent to 0.0199 bar. We can use the ideal gas law to find the number of moles of water vapor present in the 6H₂O(g) component of the equilibrium;
PV = nRT
(0.0199 bar) (V) = n (8.314 J/mol·K) (298 K)
n = 0.001535 mol
So the equilibrium constant Kp is;
Kp = (P(MCl₂)/P°) (P(H₂O)⁶/P°)
where P(MCl₂) is the partial pressure of MCl₂, P(H₂O) is the partial pressure of water vapor, and P° is the standard pressure of 1 bar. Since MCl₂ is a solid, its partial pressure is negligible and can be assumed to be zero. So we have;
Kp = (0/1 bar) (0.0199 bar)⁶/1 bar = 7.58×10⁻²⁰
Now we can calculate ΔrG;
ΔrG = -RT ln(Kp) = -(8.314 J/mol·K) (298 K) ln(7.58×10⁻²⁰) ≈ 190.4 kJ/mol
Therefore, ΔrG is approximately 190.4 kJ/mol when the pressure of water vapor is 19.9 mbar.
To find ΔrG∘, we need to use the relationship between ΔrG∘, Kp∘, and the standard state Gibbs energy of formation of the reactants and products;
ΔrG∘ = -RT ln(Kp∘) = ΣnΔfG∘(products) - ΣnΔfG∘(reactants)
where ΔfG∘ is the standard state Gibbs energy of formation of the species and n is the stoichiometric coefficient.
We can assume that the standard state of the solid MCl₂ is the same as that of its constituent elements M and Cl₂, which is zero. The standard state of water vapor is also assumed to be zero. So we have;
ΔrG∘ = 0 - [ΔfG∘(MCl₂) + 6ΔfG∘(H₂O)] = -6ΔfG∘(H₂O)
We can use the relationship between vapor pressure and Gibbs energy of vaporization to find ΔfG∘(H₂O);
ln(P/P°) = -ΔvapH∘/RT + ΔfG∘(H₂O)/RT
where P is the vapor pressure, P° is the standard pressure of 1 bar, ΔvapH∘ is the standard enthalpy of vaporization of water (40.7 kJ/mol), and R is the gas constant.
At the boiling point of water (100°C or 373 K), the vapor pressure is equal to 1 bar. So we have;
ln(1 bar/1 bar) = -40.7 kJ/mol/(8.314 J/mol·K)(373 K) + ΔfG∘(H2O)/(8.314 J/mol·K)(373 K)
ΔfG∘(H₂O) ≈ -57.8 kJ/mol
Therefore, ΔrG is -57.8 kJ/mol when the pressure of water vapor is 1 bar.
To know more about vapor pressure here
https://brainly.com/question/14617982
#SPJ4
PLEASE HELP FAST !!!
How should measurement of car 2 be taken to accurately measure the distance it travels? Be sure to include where the meter stick should be placed.
the experimental setup, with car 1 is at the top of a ramp and car 2 at the bottom of the ramp
car 1
/ ←top of ramp
/ ←bottom of ramp
car 2
Answers
answer
The meter stick should be placed at the starting position of car 2 at the base of the ramp so that it measures the part of car 2 that will end up the furthest away from the base of the ramp.
explanation
i dont know if this is right or not but my teacher gave me a 90 so there is a 50/50 chance its right or wrong
A certain amount of NaOH is dissolved in certain kilograms of solvent and molality of the solution is 0.5 m. When the same amount of NaOH is dissolved in 100 grams of less solvent than initial then molality becomes 0.625 m. Determine the amount of NaOH and the initial mass of solvent.
Answers
\(\sf\bold{❍ Given:-}\)
NaOH is dissolved in certain kilograms of solvent and molality of solution 0.5m.
Again , same among of NaOH is dissolved in 100 grams of solvent than initial , then molality becomes 0.625m.
$\space$
Now lets find the amount of NaOH and the initial mass of solvent.
Let,
$\sf\small{Initial\:Mass\:of\:solvent=y}$$\sf\small{Number\:of\:moles\:NaOH\:dissolved=x}$$\space$
$\sf\bold{ ❍ We\:know,}$
$\sf{Molality(m)=}$ $\sf\dfrac{No.of\:moles\:of\:solute}{No.of\:solvent\:in\:kg}$
$\space$
$\sf\bold{Putting\:the\:formula:-}$
$\space$
$\sf\huge\underline\bold{ ❍Case:1}$
$\space$
$\longmapsto$ $\sf\small{0.5}$ $\sf\dfrac{x}{y}$
$\space$
$\longmapsto$ $\sf{0.5y = x }$
$\space$
$\longmapsto$ $\sf{multiply\:by\:2→ y = 2x}$
$\space$
$\sf\huge\underline\bold{ ❍Case:2}$
$\space$
$\longmapsto$ $\sf\small{0.625}$ $\sf\small\dfrac{x}{y}$ = $\sf\dfrac{x}{y=100g}$
$\space$
$\longmapsto$ $\sf\small{0.625}$ $\sf\dfrac{x}{y-100/1000kg}$ = $\sf\dfrac{x}{y-0.1kg}$
$\space$
$\longmapsto$ $\sf{0.625(y-0.1kg)=x}$
$\space$
$\longmapsto$ $\sf{0.625y-0.0625=x}$
$\space$
$\sf\small\bold{By\:putting\:the\:value\:of \:"x" we\: get:}$
$\space$
$\longmapsto$ $\sf{0.625(2x)-0.0625 = x}$
$\space$
$\longmapsto$ $\sf{1.25x 0.0625 = x}$
$\space$
$\longmapsto$ $\sf{1.25x - x = 0.0625}$
$\space$
$\longmapsto$ $\sf{0.25x = = 0.0625}$
$\space$
$\longmapsto$ $\sf\small{x=}$ $\sf\dfrac{0.0625}{0.25}$= $\sf\bold{x=0.25}$
$\space$
$\sf{So,y=2(x)=2\times0.25=}$ $\sf\bold{y=0.5}$
$\space$
$\sf\small{Initial\:mass\:of\:solvent:0.5kg=500g}$
$\space$
$\sf{Now,}$
Amount of NaOH=
$\space$ $\space$ $\space$ $\space$ $\space$ $\sf{=x\times molar\:mass}$
$\space$ $\space$ $\space$ $\space$ $\space$ $\sf{=0.25\times 40=10}$
$\space$
$\sf\underline{\underline{ ⚘ Hence,amount\:of\:NaOH=10kg}}$
_______________________________
why do you think land is considered a resource
Answers
Answer:
Land is considered as an important resource as it provides habitation to a wide variety of flora and fauna. It is also used by human beings for various purposes such as agriculture, forestry, mining, building houses and roads, and setting up industries.
Chemists can identify the composition of some unknown salts by conducting a flame test. When potassium salts are heated in a flame, a purple color is observed.
This is due to the movement of electrons between energy levels. What is the electron configuration of a potassium atom at ground state?
answer choices
1s2; 2s2; 2p6; 3s2; 3p6; 4d1
1s2; 2s2; 2p6; 3s2;3p6; 3d1
1s2; 2s2; 2d6; 3s2; 3d6; 4s1
1s2; 2s2; 2p6; 3s2; 3p6; 4s1
Answers
A potassium atom's ground state electron configuration is 1s2, 2s2, 2p6, 3s2, 3p6, 4s1.
What substance is electronic configuration 1s2 2s2 2p6 3s2 3p6 4s1?An atom's electron configuration is a picture of how electrons are arranged in relation to orbital shells and subshells. Consequently, this is potassium's electron configuration.
How can you express a whole electron configuration in writing?Making Electron Configurations in Writing. Write the energy level (the period) first, then the subshell that needs to be filled, and finally the superscript, which indicates how many electrons are in that subshell. The atomic number, Z, is the sum of all the electrons.
To know more about electron configuration visit:-
https://brainly.com/question/29757010
#SPJ4
How many grams of lithium are in 1.25 x 10^26 atoms of Li?
Answers
Mass of Lithium = 1443.73 g ≈ 1.4 kg
Further explanationThe mole is the number of particles(molecules, atoms, ions) contained in a substance
1 mol = 6.02.10²³ particles
Can be formulated
N=n x No
N = number of particles
n = mol
No = Avogadro's = 6.02.10²³
The number of atoms of Li = N=1.25 x 10²⁶
mol Li(n) :
\(\tt n=\dfrac{N}{No}\\\\n=\dfrac{1.25\times 10^{26}}{6.02\times 10^{23}}\\\\n=2.08\times 10^2\)
mass Li (Ar=6.941 g/mol)
\(\tt mass=mol\times Ar\\\\mass=208\times 6,941\\\\mass=1443.73~g\)
before approving a new drug, the u.s. food and drug administration requires that companies submit the results of rigorous scientific testing on the drug’s effectiveness. these results are reviewed by physicians, statisticians, chemists, pharmacologists, and other scientists who use logic to determine whether or not a drug is safe.
Answers
The given statement is true. Scientists who use logic to determine whether or not a drug is safe. These researchers are using the classical model of decision making.
A logical and methodical approach to decision-making is the classical model of decision-making. This model proposes that decision-makers gather all relevant data, thoroughly evaluate the alternatives, then select the alternative that maximises their utility or produces the desired result. This model makes the supposition that decision-makers have full knowledge of all available options, are unbiased and logical in their assessment, and make choices free from prejudice or emotional influence. It employs a sequential procedure that involves recognising the issue, specifying the decision criteria, allocating weight to each, producing alternatives, assessing the alternatives, and selecting the best option.
To know more about classical model of decision making, here:
https://brainly.com/question/33492816
#SPJ4
What do you call a question that can be answered through an investigation
Answers
Answer:
Indepent variable
Answer:
Scientific Question. A scientific question can be answered through an investigation. What is tested? Scientists call this an independent variable.
Explanation:
Calculate the power developed in R1.
P1 =_____watts
48
190
240
1300
Answers
the answer will be 190
Explanation:
because R1= 0.5*0.5*8
=190
Ashley determines the density, mass, and volume of a sample of liquid water. She removes part of the sample and repeats her measurements with the remaining water. Which of the following properties should have the same value for both of her measurements?
○All of the above
○Density
○Mass
○Volume
Answers
Answer:
Only thing that should hold constant is the Density of the water
Explanation:
Since you are removing some of the water, the Mass and Volume will decrease.
Density of the water are the properties should have the same value for both of her measurements.
What is density ?The density can be described as the mass per unit volume like it is the ratio between mass and volume, how much stuff an object has in a unit volume.
Density is a determining factor of matter, where it shows how tightly the matter is crammed together, the term was discovered by the Greek scientist Archimedes
The density can be calculated as the mass (m) can be divided by the volume (v)
Density = Mass/Volume
The SI unit of density is kilogram per cubic meter (kg/m3).
Learn more about density, here:
brainly.com/question/15164682
#SPJ2
The results of the gold foil experiment led to the
conclusion
that an atom is
A) mostly empty space and has a small, negatively
charged nucleus
B) mostly empty space and has a small, positively
charged nucleus
C) a hard sphere and has a large, negatively charged
nucleus
D) a hard sphere and has a large, positively charged
nucleus
Answers
Answer:
B) mostly empty space and has a small, positively charged nucleus
Explanation:
In the gold foil experiment, positively-charged alpha particles were directed towards a gold foil sheet. During the experiment, most of the particles went through the gold foil. However, a select few alpha particles were met with resistance and bounced off the sheet.
This proves that the gold atoms, which made up the gold foil sheet, were mostly empty space as most of the alpha particles passed through it. Furthermore, the particles which bounced off the sheet must have hit small, positively-charged nuclei. The nuclei must have been positive because similar charges repel each other. In other words, if the nuclei were negatively-charged, the positively-charged alpha particles would not bounce off the sheet, but instead "stick" to it.
Lithium stearate (octadecanoate) is made by neutralising stearic acid (octadecanoic
acid) with lithium hydroxide.
CH,(CH₂) 16COO Li* + H₂O
LiOH + CH,(CH₂) 16COOH
It is used as a component of low melting point greases that can be used down to
-60°C. In an experiment, 0-048 mole of stearic acid was neutralised by a lithium
hydroxide solution of concentration 0-64 mol dm
Calculate the volume of this lithium hydroxide solution needed to neutralise the
0-048 mole of stearic acid.
[2]
0.048 mole of
Stearic acid
Conc of LOH = 0.64 moi dm
-3
R = 1:1
:: 0.048 =
(1
clu
0.048
0.64
LOH Mole
=
0.075 dm-3
0.075
Answers
Explanation:
Yes, that is correct. The formula weight of a compound is the sum of the atomic weights of all the atoms in the compound's chemical formula. To determine the percentage by mass of an element in a compound, you can use the molar mass of that element and divide it by the formula weight of the compound, then multiply by 100. In the case of a hydrated compound, the mass of the water molecules will dilute the percentage of the other elements, resulting in a lower percentage for the element of interest.
The reaction conditions for a specific reaction, bcd ha ⟶⟶ abcdh, are correct, but the reaction does not occur. what could be the reason?
Answers
The reason for the reaction does not occur is the orientation of the molecule formed with respect to each other is not correct for the reaction to occur
ORIENTATIONIn chemical reaction the collision between the atom, the reactant molecules must collide with favorable orientation ensure the direct contact between the atom that involved in breaking and forming the bond.
ORIENTATION OF THE MOLECULEThe extension of macromolecule, the structural units along the fiber axis that determines the sorption of solvent, tensile strength, optical behaviors and modulus
Hence, the reason for that reaction does not occur is the orientation of the molecule formed with respect to each other is not correct for the reaction to occur.
Learn more about the orientation on
https://brainly.com/question/12093393
#SPJ4
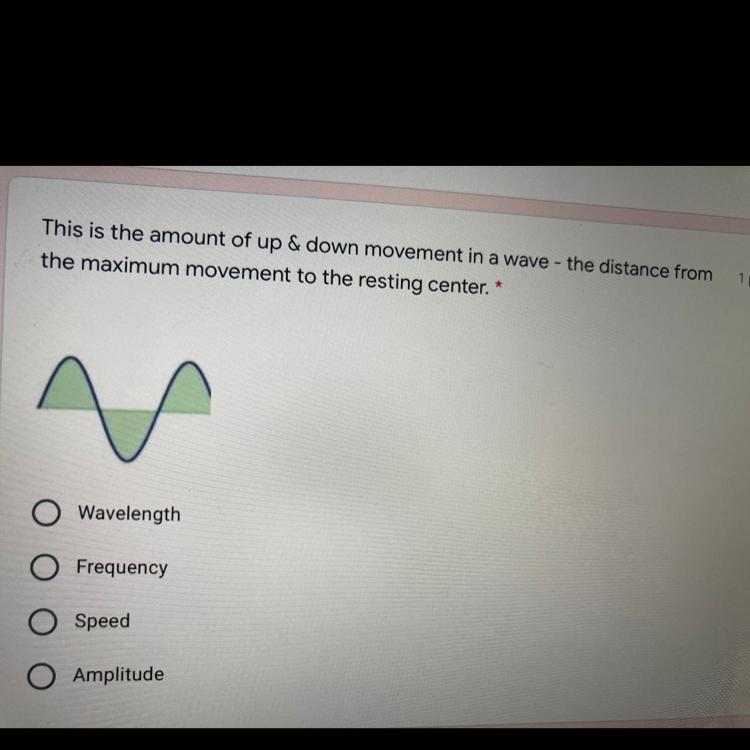
Please help fasts thanks
Answers
Answer:
amplitude
Explanation:
The amplitude of a wave is the distance from the centre line (or the still position) to the top of a crest or to the bottom of a trough
Answer:
I think it is wave length
Explanation:
yeah pretty sure