A heavily wooded area needs additional solar collectors to meet energy demand. Which statement describes a possible environmental consequence of adding these collectors?
A.
The amount of air pollution will increase.
B.
The amount of water pollution will increase.
C.
More trees will need to be planted to insulate the solar collectors.
D.
Trees will need to be cleared for the solar collectors to work efficiently.
Answers
Answer:
C
I think not positive good luck tho
A heavily forested area is one that has a dense and large number of trees. The areas like mountainous tracks or the protected frosts areas of wildlife.
In order to have solar collectors that meet the demands of energy generation. Some parts of the forests may have to clear off. As for collecting the sunlight, there needs to be clear ground. As trees and forests absorb insolation.Hence the option D is correct, trees need to to be cleared for working efficiently.
Learn more about the area that needs additional solar collectors to meet energy demand.
brainly.in/question/40583530.
Related Questions
The average bond dissociation energy of a carbon-carbon bond is 410 kj/mol. What wavelength in nanometers of ultraviolet radiation has an energy of 410 kj/mol?.
Answers
Wavelength in nanometers of ultraviolet radiation has an energy of 410 kj/mol is 194nm
Wavelength is the distance between identical points means adjacent crests in the adjacent cycles of a waveform signal propagated in space or along a wire
Here given data is average bond dissociation energy of a carbon-carbon bond is = 410 kj/mol and ultraviolet radiation has an energy of 410 kj/mol
We have to find wavelength in nanometers = ?
We have formula
λ = hc/E where, h=plank constant
λ = 6.63×10⁻³⁴×410/410
λ = 1.941×10⁻³⁴
So,1nm = 10⁻⁹m
1.941×10⁻³⁴m = 194nm
λ = 194nm
Know more about wavelength
https://brainly.com/question/13476709
#SPJ1
Suppose you see a crescent Moon. How do you know if it is waxing or waning?
Answers
Answer:
waxing is in the evening and waning is in the morning if you cant see the moon in the evening it is waning if you can see it in the evening it is waxing
lewis model of electronic structure key questions 1. what distinguishes each of the four examples in illustrating the methodology? 2. why might it be necessary to put double or even triple bonds between atoms in constructing lewis structures? 3. how does the lewis structure help you identify the length of bonds in a molecule? 4. how is formal charge determined, and how is it used in identifying reasonable lewis structures? 5. in the above examples illustrating the methodology, why are relevant resonant structures - present only in the case of no2 ? 6. why can c, n, o, and f accommodate only eight electrons when in a molecule while other atoms, such as i, can accommodate more than eight?
Answers
1. Each example in illustrating the Lewis model methodology is distinguished by the specific arrangement and bonding of atoms within the molecule. 2. Double or triple bonds may be necessary in constructing Lewis structures to satisfy the octet rule and achieve a more stable electron configuration. 3. The Lewis structure helps identify the length of bonds in a molecule by considering the number of shared electron pairs between atoms. 4. Formal charge is determined by comparing the number of valence electrons an atom has in a Lewis structure with its actual electron count, and it is used to identify reasonable Lewis structures by minimizing formal charges. 5. Relevant resonant structures are present only in the case of NO2 due to the presence of delocalized pi bonds and the ability to distribute electrons among multiple bonding arrangements. 6. C, N, O, and F can accommodate only eight electrons in a molecule due to their small atomic size and high electronegativity, whereas larger atoms like I can accommodate more than eight electrons due to the presence of empty d orbitals.
1. The four examples in illustrating the methodology of the Lewis model of electronic structure are distinguished by the specific elements and their arrangements in the molecules or ions being considered .
2. It might be necessary to put double or even triple bonds between atoms in constructing Lewis structures to satisfy the octet rule, which states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight valence electrons .
3. The Lewis structure helps identify the length of bonds in a molecule through the concept of bond order. In general, a higher bond order (resulting from multiple bonds) corresponds to a shorter bond length, as multiple bonds are stronger and hold the atoms closer together.
4. Formal charge is determined by comparing the number of valence electrons an atom would have in an isolated state with the number of electrons assigned to it in a Lewis structure. It is used in identifying reasonable Lewis structures by helping to evaluate the distribution of charge and stability of different resonance structures or electron arrangements.
5. Relevant resonant structures are present only in the case of NO2 because nitrogen dioxide (NO2) exhibits resonance, where the electrons in the molecule can be delocalized between multiple bonding arrangements. Resonance structures help explain the bonding and stability of molecules that cannot be adequately represented by a single Lewis structure [relevant resonant structures, NO2, illustrating the methodology].
6. Carbon (C), nitrogen (N), oxygen (O), and fluorine (F) can accommodate only eight electrons in a molecule due to their small atomic sizes and high electronegativities. These atoms have a strong tendency to achieve a stable electron configuration by gaining or losing electrons to complete their valence shells. In contrast, larger atoms like iodine (I) can accommodate more than eight electrons because they have more available orbitals for electron bonding [C, N, O, F, accommodate eight electrons, other atoms, iodine].
Learn more about Lewis model from the given link
https://brainly.com/question/33416282
#SPJ11
On a hot day, a student places a glass of cold lemonade on a table outdoors. After a few minutes, water droplets have formed on the outside of the glass.
Is energy absorbed or released by the cold lemonade? Explain your answer.
Compare average kinetic energy for the air molecules and lemonade molecules when the student first places the lemonade outdoors. Explain your answer.
Explain how and why the water droplets form on the outside of the glass.
Answers
Answer:
ye but sdfajkd tryng to play me
Explanation:
Answer:
Vapor is released out of the lemonade because when you place it in a hot it will increase.
Explanation:
A student is working with four beakers that each contains a clear liquid. Which set of procedures would be best to use to determine whether one of the beakers contains only distilled water?
Group of answer choices
Observe volume, determine mass, observe color, determine pH
Observe odor, determine temperature, observe color, determine boiling point
Observe odor, determine pH, determine density, determine boiling point
Determine mass, observe volume, determine temperature, observe odor
Answers
Answer:
Observe odor, determine pH, determine density, determine boiling point
Explanation:
The correct procedures that would be best to use to determine whether a beaker contains only distilled water would be to observe the odor of the liquid in the beaker, determine the pH of the liquid, determine the density, and then determine the boiling point of the liquid.
Water is generally odorless and has a pH of approximately 7 with a density of 1 kg/m3 and a boiling point of 100 \(^oC\). If the liquid in the beaker ticks all these conditions, then it can be established to be only distilled water.
The width of a spectral line of wavelength 300 nm is measured as 0. 01 nm. What is the average time that the system remains in the corresponding energy state?
Answers
Therefore, the average time that the system remains in the corresponding energy state is equal to or greater than 0.005 x 10^(-9) seconds.
To calculate the average time that the system remains in the corresponding energy state, we can use the uncertainty principle.
The uncertainty principle states that the product of the uncertainty in the measurement of position (∆x) and the uncertainty in the measurement of momentum (∆p) must be greater than or equal to the reduced Planck's constant (ħ):
∆x ∆p ≥ ħ
In the case of a spectral line, the uncertainty in wavelength (∆λ) can be related to the uncertainty in momentum (∆p) using the relation ∆p = ħ / ∆λ.
Given that the width of the spectral line is measured as 0.01 nm, we can convert it to meters by multiplying by 10^(-9) (since 1 nm = 10^(-9) m):
∆λ = 0.01 nm = 0.01 x 10^(-9) m
Substituting this into the relation ∆p = ħ / ∆λ, we have:
∆p = ħ / (0.01 x 10^(-9) m)
Now, the uncertainty in momentum (∆p) can be related to the average time (∆t) using the relation ∆p ∆t ≥ ħ/2.
∆p ∆t ≥ ħ/2
Substituting the value of ∆p, we have:
(ħ / (0.01 x 10^(-9) m)) ∆t ≥ ħ/2
Simplifying, we find:
∆t ≥ (0.01 x 10^(-9) m) / 2
∆t ≥ 0.005 x 10^(-9) s
Therefore, the average time that the system remains in the corresponding energy state is equal to or greater than 0.005 x 10^(-9) seconds.
Learn more about energy state here
https://brainly.com/question/28333978
#SPJ11
How are nadh and nadph similar and how do they differ? describe roles of each of these cofactors in human metabolism.
Answers
While the lower ribose ring's number two carbon in NADPH's structure has a phosphate, which alters the binding capabilities, NADH's structure is identical to that of NADPH.
While NADPH often serves as a reductant for anabolic processes, AND+ typically acts as an oxidant for catabolic reactions.
How do NADH and NADPH compare and contrast one another?Coenzymes NADPH and NADH participate in a number of metabolic activities.
A second phosphate group is present in NADPH. While NADPH is involved in photosynthesis, NADH is involved in cellular respiration.
The reduced versions of NADP+ and NAD+ are NADPH and NADH, respectively.
What function do NADPH and NADH serve in metabolism?The nicotinamide adenine dinucleotide (NAD+)/reduced NAD+ (NADH) and NADP+/reduced NADP+ (NADPH) redox couples are crucial for preserving cellular redox homeostasis and for controlling a variety of biological processes, including cellular metabolism.
What roles does NADPH play?A coenzyme called NADPH provides electrons to a variety of cellular processes.
learn more about metabolism here
https://brainly.com/question/1490181
#SPJ4
Which sample produced the higher amount of radiation? O The sample with an activity of 8 kBq O The sample with an activity of 17 mCi
Answers
The sample with an activity of 17 mCi (629,000,000 Bq) produced a higher amount of radiation than the sample with an activity of 8 kBq (8,000 Bq).
To determine which sample produced the higher amount of radiation, we'll need to compare the activities of the two samples:
1. The sample with an activity of 8 kBq (kilo-Becquerels)
2. The sample with an activity of 17 mCi (milli-Curies)
First, let's convert both activities to a common unit, Becquerels (Bq).
1 kBq = 1,000 Bq
1 Ci = 37,000,000,000 Bq (37 billion Bq)
1 mCi = 0.001 Ci
Now, let's convert the activities:
- Sample 1: 8 kBq = 8,000 Bq
- Sample 2: 17 mCi = 17 * 0.001 Ci = 0.017 Ci = 0.017 * 37,000,000,000 Bq = 629,000,000 Bq
Comparing the activities, we find that the sample with an activity of 17 mCi (629,000,000 Bq) produced a higher amount of radiation than the sample with an activity of 8 kBq (8,000 Bq).
Learn more about radiation at brainly.com/question/13934832
#SPJ11
which of these is true about the angle of incidence and angle of reflection

Answers
I used photo math
Which units express heat capacity? J/°C, J/K, cal/°C, cal/K J/(gi°C), J/(giK), cal/(gi°C), cal/(giK) J, cal °C, K
Answers
Answer:
a
Explanation:
The heat capacity of a substance is the heat energy required to rise its temperature per one degree Celsius. Hence its unit is J/°C.
What is heat capacity ?Heat capacity is the amount of heat energy required to raise the temperature of a substance by 1 degree Celsius or 1 Kelvin. It is expressed in the following units:
Joules per degree Celsius (J/°C)
Joules per Kelvin (J/K)
Calories per degree Celsius (cal/°C)
Calories per Kelvin (cal/K)
Joules per gram per degree Celsius (J/(g·°C))
Joules per gram per Kelvin (J/(g·K)) etc.
If in terms of simply the energy, then, The following units are used.
Joules (J) , Calories (cal) , Degrees Celsius (°C), Kelvin (K)
The choice of unit depends on the specific application and the system of units being used. The SI unit for heat capacity is J/K, while the traditional unit is cal/°C.
The use of per gram units is common in the context of specific heat capacity, which is the amount of heat energy required to raise the temperature of a unit mass of a substance by 1 degree Celsius or 1 Kelvin.
Therefore, here, the unit of heat capacity is J/°C.
Find more on heat capacity :
https://brainly.com/question/28302909
#SPJ7
1. What type of TV uses a CCFL for backlighting?
O 3D
O LCD
OLED
O Cathode Ray tube
Answers
Explanation:
When a virus infects a bacterial cell, often new viruses are assembled and released when the host bacterial cell is lysed. If these new viruses go on to infect new bacterial cells, the new host cells may not be lysed. What is the most plausible explanation for this?A) The bacterial cell must be resistant to infection by the virus.
B) The virus carries genes that confer resistance to the host bacterial cell.
C) The host bacterium couples the viral infection with transformation.
D) The virus has entered the genome of the bacterial cell and is in the lysogenic stage.When a virus infects a bacterial cell, often new viruses are assembled and released when the host bacterial cell is lysed. If these new viruses go on to infect new bacterial cells, the new host cells may not be lysed. What is the most plausible explanation for this?A) The bacterial cell must be resistant to infection by the virus.B) The virus carries genes that confer resistance to the host bacterial cell.
C) The host bacterium couples the viral infection with transformation.
D) The virus has entered the genome of the bacterial cell and is in the lysogenic stage.Answer:
LCD TV's use a CCFL for backlighting
Calcium carbonate is what kind of compound?
Answers
Answer:
Chemical Compound
Explanation:
I'm not sure why but I know it's chemical. Hope this helps!
30 POINTS
do you think snow is an ionic compound or a covalent compound? explain
Answers
Answer:
Compounds containing two elements (so called binary compounds) can either have ionic or covalent bonding. If a compound is made from a metal and a non-metal, its bonding will be ionic. If a compound is made from two non-metals, its bonding will be covalent.
Explanation:
Can u mark brainliest please luvss!!
How does protein substance in an animal hide or skin be prevented from bacterial infection during the process of tanning? * a.By soaking in water b.By deliming using an acid c.By brine curing d.By scudding with dull knife
Answers
Answer:
c. By brine curing
Explanation:
Tanning refers to the process of treating the hides and skins of animals to produce leather.The different processes involved in the tanning of leather include:
Curing: this is the process of applying salt to the animal hide to prevent decay from bacterial growth. It also serves to reduce the moisture content of the animal hide.
Soaking : this involves soaking the hides in clean water to remove the salt left over from curing and increase the moisture content for further treatment of the hide.
Liming: Liming is done by applying lime to remove hair, fat and grease and in order for the hide to swell up.
Scudding: This is the process of further removal of usually done first by a machine, and then manually using a dull knife.
Deliming: This is done using an acid to reduce the pH of the collagen found in the hide being tanned.
In semiconductor manufacturing, wet chemical etching is often used to remove silicon from the backs of wafers prior to metallization. The etch rate is an impor
Answers
Sample means for solutions 1 and 2 are 19.27 and 10.32 respectively
In semiconductor manufacturing,
The total for answer 1 is given by:
9.7+10.5+9.4+10.6+9.3+10.7+9.6+10.4+10.2+10.5 = 192.7
The sample size is 10 and provides us with
192.7/10 = 19.27
For solution 2, the sum is given by:
10.6+10.3+10.3+10.2+10.0+10.7+10.3+10.4+10.1+10.3 = 103.2
The sample size is 10, this gives us
103.2/10 = 10.32
The total for answer 2 is given by:
10.6+10.3+10.3+10.2+10.0+10.7+10.3+10.4+10.1+10.3 = 103.2
The sample size is 10 and provides us with
103.2/10 = 10.32
Learn more about semiconductor manufacturing here brainly.com/question/22779437
#SPJ4.
In semiconductor manufacturing, wet chemical etching is often used to remove silicon from the backs of wafers prior to metalization. The etch rate is an important characteristic in this process and is known to follow a normal distribution. Two different etching solutions have been compared, using two random samples of 10 wafers for each solution. Assume the variances are equal. The etch rates are as follows (in mils per minute): Solution 1 Solution 2 9.7 10.6 10.5 10.3 9.4 10.3 10.6 10.2 9.3 10.0 10.7 10.7 9.6 10.3 10.4 10.4 10.2 10.1 10.5 10.3 Calculate sample means of solution 1 and solution 2
If a solution is saturated and more salt that is added falls to the bottom of the container what is it called?
A: Supersaturated
B: Saturated
C: Unsaturated
Answers
Answer:
The answer is B
Explanation:
A solution that contains a relatively low concentration of solute is called dilute, and one with a relatively high concentration is called concentrated. If we add more salt to a saturated solution of salt, we see it fall to the bottom and no more seems to dissolve.
A tin of chopped tomatoes weighs 145 grams.
You buy four of these tins.
What is the total mass of these four tins
i in grams
cont.
b
ii in kilograms?
Answers
The total mass of 4 tin is in grams is 580 grams and in kg is 0.58kg.
One tin of chopped tomatoes weight is 145 grams
and we have to calculate it for 4
so for 4
145*4 =580 grams
and we have to change it into kilograms so
580 /1000 = .58kg
By 1,000, divide the number of grams.
To convert from grams to kilograms, simply divide by 1,000 because there are 1,000 grams in every kilogram. If necessary, construct the equation using fractions. Put the number of grams over 1 and divide it by the conversion factor, which is 1 kg / 1,000 g. Your answer in kilograms will result from the cancellation of the grams. Remember to use the appropriate units to mark your response.
know more about grams to kilograms click here;
https://brainly.com/question/12065899
How is a joule defined?
A. It is the work done when a force of 1 newton is applied to an object for a distance of 1 meter.
B. It is the force needed to make an object with a mass of 1 kilogram accelerate by 1 kilogram per second squared.
C. It is the force needed to make an object with a mass of 1 kilogram travel 1 meter.
D. It is the work done when a force of 1 newton is applied to accelerate an object by 1 kilogram per second squared.
Answers
Answer:
A. It is the work done when a force of 1 newton is applied to an object for a distance of 1 meter
Explanation:
The work done when a force of 1 newton is applied to an object for a distance of 1 meter is called as joule. Therefore, option A is correct.
What do you mean by work done ?The term work done is defined as whenever work is done, energy is transferred.
To move an object, it must be converted into energy. The method of force can be used to transfer energy. Work done is the amount of energy transferred by a force to move an object.
Work can be calculated using the formula: Work = Force Distance. The joule (J) or Newton meter (N m) is the SI unit for work. One joule is the amount of work done when one Newton of force moves an object one meter.
Thus, The work done when a force of 1 newton is applied to an object for a distance of 1 meter is called as joule, option A is correct.
To learn more about the work done, follow the link;
https://brainly.com/question/13662169
#SPJ2
HELP FAST
H₂S gas is removed from the system at
equilibrium below. How does the
system adjust to reestablish
equilibrium?
NH4HS(s) = NH3(g) + H₂S(g)
A. The reaction shifts to the right (products) and the
concentration of NH3 decreases.
B. The reaction shifts to the left (reactants) and the
concentration of NH3 decreases.
C. The reaction shifts to the right (products) and the
concentration of NH3 increases.
D. The reaction shifts to the left (reactants) and the
concentration of NH3 increases.
Answers
When H₂S gas is removed from the system at equilibrium, the reaction shifts to the right (products) and the concentration of NH₃ increases (option C)
How do i determine where the reaction will shift to?A French scientist (Chatelier) postulated a principle which helps us to understand a chemical system in equilibrium.
The principle states that If a an external constraint such as change in temperature, pressure or concentration is imposed on a system in equilibrium, the equilibrium will shift so as to neutralize the effect.
According to Chatelier's principle a decrease in concentration of the products will favor the forward (right) reaction.
From the above principle, we can conclude that when H₂S gas is removed from the system at equilibrium, the reaction shifts to the right (products) and the concentration of NH₃ increases.
Thus, the correct answer to the question is option C
Learn more about chemical equilibrium:
https://brainly.com/question/4289021
#SPJ1
Which statements about the behavior of gaseous H2 molecules in a container at 1 atm and 298 K are correct?
I. All H2 molecules are moving at the same speed.
II. The H2 molecules are colliding more frequently with the walls of the container than they would in the same container at 398 K.
One, both, or neither?
Answers
The correct statement about the behavior of gaseous H₂ molecules in a container at 1 atm and 298 K is: (II) The H₂ molecules are colliding more frequently with the walls of the container than they would in the same container at 398 K.
Statement I is incorrect. In a gas at a given temperature, the molecules have a range of speeds following a distribution known as the Maxwell-Boltzmann distribution. Not all H₂ molecules are moving at the same speed. Instead, they have a distribution of speeds, with some moving faster and some moving slower.
Statement II is correct. According to the kinetic theory of gases, the frequency of molecular collisions with the walls of the container is directly related to the temperature.
At a lower temperature of 298 K compared to 398 K, the H₂ molecules will collide with the walls of the container more frequently because their average kinetic energy and speed will be lower.
To know more about the Maxwell-Boltzmann distribution refer here :
https://brainly.com/question/31833642#
#SPJ11
Our un i a tar that i fueled by a pecific type of nuclear reaction. Which type of nuclear reaction i thi
Answers
Our sun is a star that is fueled by a specific type of nuclear reaction. The type of nuclear reaction is Fusion.
When two atoms collide to create a heavier atom, such as when two hydrogen atoms combine to create one helium atom, this process is known as fusion. The Sun is a main-sequence star, and thus generates its energy by nuclear fusion of hydrogen nuclei into helium.
This process generates enormous amounts of energy, many times more than fission, and powers the sun. Furthermore, it doesn't generate radioactive fission products.
Scientists are studying fusion processes, but they are challenging to maintain for extended periods of time due to the extreme pressure and temperature required to fuse the nuclei together.
Learn more about fusion https://brainly.com/question/12701636
#SPJ4
An atom of silicon has a mass number of 28 and an atomic number of 14. How many protons are in this atom?.
Answers
The atomic number states the number of protons, so the number of protons in the element silicon is 14.
Atomic StructureAtoms are made up of subatomic particles namely protons, electrons and neutrons. In an atom the three particles have a certain amount.
Protons are particles that make up atoms that are positively charged and have a mass as large as that of hydrogen, which is 1.67262 x 10⁻²⁷ kg or 1,836 times heavier than electrons. Protons are so deep in the atomic nucleus that they cannot be disturbed by particles outside the atom.
The element silicon (Si) has an atomic number = 14 and a mass number = 28
So that the number of subatomic particles is:
The number of protons = atomic number
= 14
Learn more about protons here: https://brainly.com/question/1805828#SPJ4
Which of the following statements is FALSE?a. AgCl is predicted to be more soluble in pure water than in 0.10 M HClb. A saturated aqueous solution of AgCl is predicted to exhibit an approximately neutral pH at 25°Cc. Ag2CO3 is predicted to be more soluble in pure water than in 0.10 M HCld. AgCl is predicted to be more soluble in 0.10 M HCN than in pure water (Kf of Ag(CN)2− = 3 x 1020)
Answers
The FALSE statement among the given options is (b) A saturated aqueous solution of AgCl is predicted to exhibit an approximately neutral pH at 25°C.
When AgCl dissolves in water, it reacts with water molecules to form H+ and OH- ions, which leads to an acidic solution. Therefore, a saturated aqueous solution of AgCl is predicted to exhibit an acidic pH, not a neutral pH.Option (a) is true because AgCl is more soluble in pure water than in 0.10 M HCl due to the common-ion effect. Option (c) is false because Ag2CO3 is more soluble in 0.10 M HCl than in pure water. Option (d) is true because the formation of the complex ion Ag(CN)2− increases the solubility of AgCl in the presence of excess CN-.
To learn more about AgCl:
https://brainly.com/question/17102479
#SPJ11
suppose that 4.02 g of a silver salt ( agx ) is dissolved in 585.0 ml of water. a current of 3.31 a , applied for 875 s , is required to plate out all of the silver in solution. what is the mass percentage of silver in the salt?
Answers
Using Faraday's Law, we can find that the amount of silver is (3.31 A)(875 s)/(96,485 C/mol) = 0.0266 mol.
The first step is to calculate the amount of silver in the solution. Using Faraday's Law, we can find that the amount of silver is (3.31 A)(875 s)/(96,485 C/mol) = 0.0266 mol. Since the molar mass of Ag is 107.87 g/mol, the mass of silver is (0.0266 mol)(107.87 g/mol) = 2.87 g. Therefore, the mass percentage of silver in the salt is (2.87 g / 4.02 g) x 100% = 71.4%. To find the mass percentage of silver in the salt (AgX), we can follow these steps:
1. Calculate moles of silver (Ag): Use the given current (3.31 A) and time (875 s) to find moles of Ag using Faraday's Law. Moles of Ag = (3.31 A * 875 s) / (96,485 C/mol).
2. Determine molar mass of AgX: Divide the given mass of silver salt (4.02 g) by the moles of Ag calculated in step 1.
3. Calculate mass percentage: Divide the molar mass of Ag (107.87 g/mol) by the molar mass of AgX obtained in step 2, then multiply by 100.
By following these steps, you can find the mass percentage of silver in the silver salt.
To know more about Faraday's Law visit:
https://brainly.com/question/1640558
#SPJ11
What is the pH of a 6.4x10^-10 m OH- solutions
Answers
Answer:
pH: 4.80617997398
pOH: 9.19382002602
[H+]: 1.5625E-05
[OH-]: 6.4E-10
ACID
Hope it helps :)
how many moles of oxygen gas will be produced if 1.64 moles of sodium chloride was produced
Answers
of water can be electrolytically broken down to yield 5 moles or oxygen gas. 1.64x1023 = 1677.72 if 1.64 moles or sodium chloride were produced.
What does a gas mole weigh?A mole (6.021023 typical particles) of the any gas takes up 22.4L at STP (figure below). A comparison of various gases' molar volumes is shown in the image below. At STP, there exist samples containing helium (He), nitrous (N2), or methane (CH4). Each has a mole content of 1 or 6.02 1023 particles.
1.64x1023 = 1677.72
if 1.64 moles or sodium chloride were produced
How is a mole calculated?One mole of the a material is equivalent to 6,022 x 1023 molecules of that substance. The Avogadro number, also referred to as the Avogadro constant, is 6.022 x 1023. The definition of a mole can be used to the conversion between mass and particle count.
To know more about sodium chloride visit:
https://brainly.com/question/9811771
#SPJ1
Determine the partial pressure and number of moles of each gas in a 16.75L vessel at 30 degree C containing a mixture of xenon and neon gases only. The total pressure in the vessel is 7.10 atm, and the mole fraction of xenon is 0.721.
What is the partial pressure of xenon?
What is the partial pressure of neon?
What is the number of moles of xenon?
What is the number of moles of neon?
Answers
First, we will calculate the number of moles of mixture of Xenon and Neon gases.Number of moles of mixture of Xenon and Neon gases:
Let x be the mole fraction of Neon.
Therefore, (1 - x) is the mole fraction of Xenon
.Mole fraction of Neon + Mole fraction of Xenon = 1x + (1 - x) = 1x = 1 - (1 -
x = 0 + x
x = 0.279
Mole fraction of Neon = 0.279
Mole fraction of Xenon = 0.721
Number of moles of gas = (Total Pressure * Volume)/(Gas Constant * Temperature)
Number of moles of Xenon = (7.10 atm * 16.75L * 0.721)/(0.08206 * (273 + 30))
Number of moles of Xenon = 8.44 moles
Number of moles of Neon = (7.10 atm * 16.75L * 0.279)/(0.08206 * (273 + 30))
Number of moles of Neon = 3.29 moles
Now, we can calculate the partial pressure of Xenon and Neon.
Partial pressure of Xenon:
Partial Pressure of Xenon = Mole fraction of Xenon * Total Pressure
Partial Pressure of Xenon = 0.721 * 7.10 atm
Partial Pressure of Xenon = 5.12 atm
Partial pressure of Neon
Partial Pressure of Neon = Mole fraction of Neon * Total Pressure
Partial Pressure of Neon = 0.279 * 7.10 atm
Partial Pressure of Neon = 1.98 atm
Learn more about atoms at
https://brainly.com/question/33049833
#SPJ11
how does one determine a molecular formula from the epirical formula
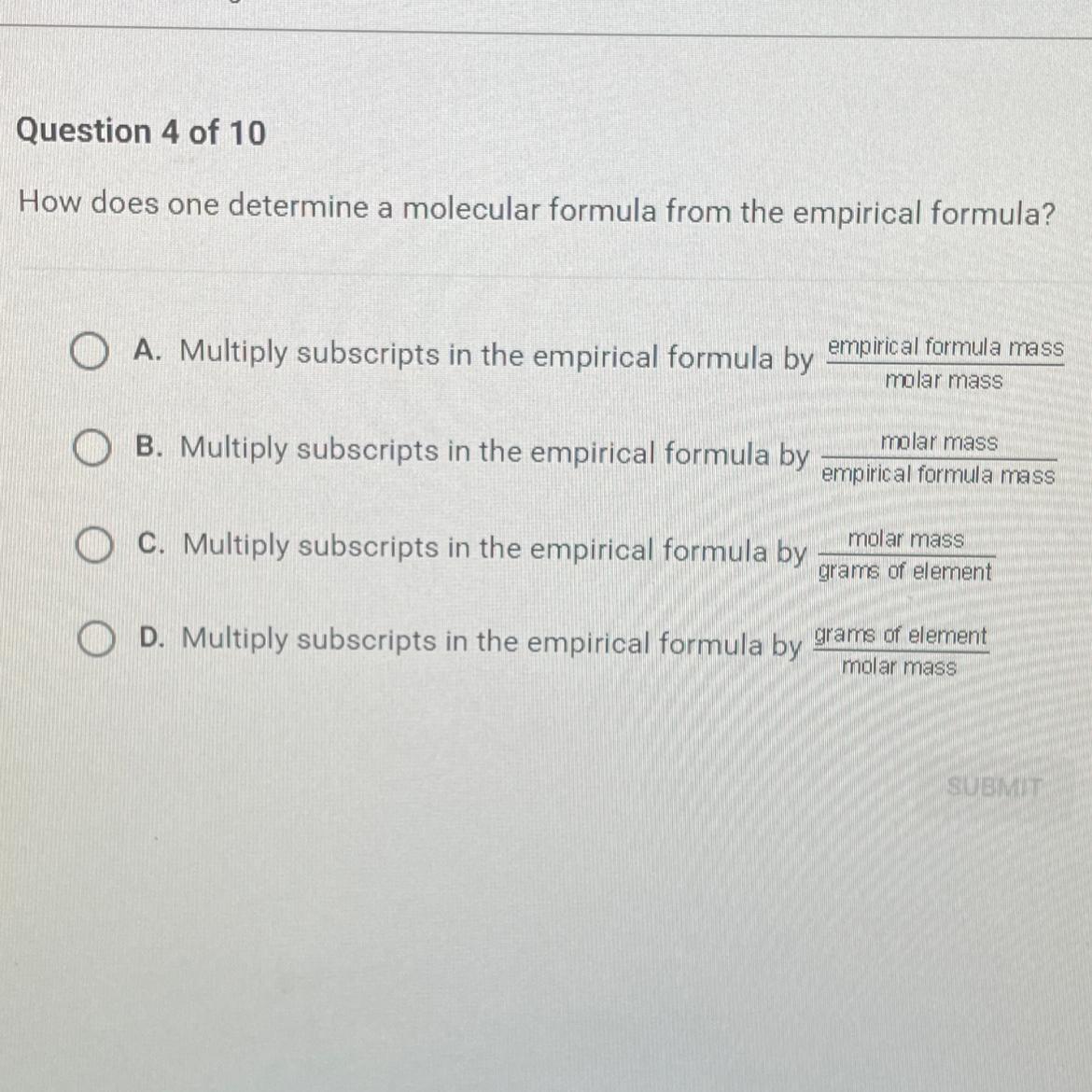
Answers
Answer:
C
Explanation:
According to the graph,
what part(s) of the
reaction are present at
the beginning of the
reaction?
Concentration (M)
Reaction: 2A A₂
A. only the reactant, A
B. only the product, A:
C. Both the reactant (A) and product (A:)
D. You cannot determine from the graph.
Time (sec)
4

Answers
According to the graph, only the reactant A was present at the beginning of the reaction.
What does the graph show?The graph shows the concentration for the reactant A and the product that is A2. In this graph, the concentration is displayed on the vertical axis, while the horizontal axis shows the time.
In general terms, it can be observed that at the beginning only the reactant A is present, but as the reaction occurs the concentration of this reactant decreases, while the concentration of the product A2 increases.
Learn more about reactions in https://brainly.com/question/30464598
#SPJ1
a water sample shows 0.064 grams of some trace element for every cubic centimeter of water. casho uses a container in the shape of a right cylinder with a radius of 8.5 cm and a height of 18.2 cm to collect a second sample, filling the container all the way. assuming the sample contains the same proportion of the trace element, approximately how much trace element has casho collected? round your answer to the nearest tenth.
Answers
According to unit conversion, there are 264.25 grams of trace element collected.
Unit conversion is defined as a multi-step process which involves multiplication or a division operation by a numerical factor.The process of unit conversion requires selection of appropriate number of significant figures and the rounding off procedure.
It involves a conversion factor which is an expression for expressing the relationship between the two units.It is defined as a substance which cannot be broken down further into any other substance. Each element is made up of its own type of atom. Due to this reason all elements are different from one another.
There are 0.064 gram in 1 cm³ of water thus in cylinder with a radius of 8.5 cm and a height of 18.2 cm volume is πr²h = 3.14×8.5×8.5×18.2=4128.94 cm³ thus this volume has 4128.94×0.064=264.25 g.
Learn more about unit conversion,here:
https://brainly.com/question/19420601
#SPJ4