A flask contains a mixture of hydrogen gas and water vapor at STP. If the pressure of the water vapor is 19.5 mmHg, then what is the pressure of the hydrogen gas?
779 mmHg
779 mmHg
760 mmHg
760 mmHg
81.7 mmHg
81.7 mmHg
741 mmHg
Answers
Answer:
I would say A but not sure
Explanation:
Related Questions
I really need this im struggling q is heat
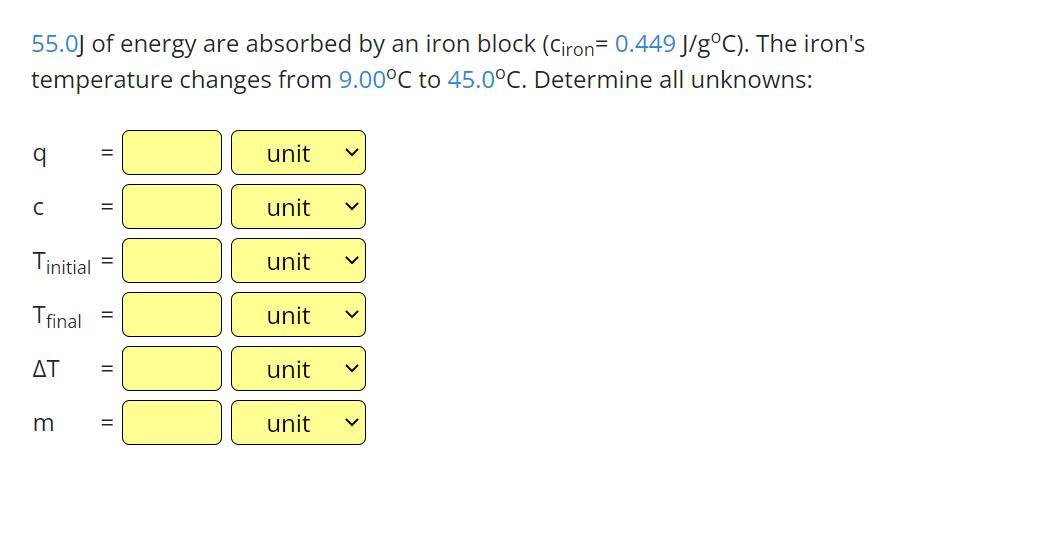
Answers
The unknown from the given question are:
Heat energy, Q = 55.0 JSpecific heat capacity, C = 0.449 J/gºC Initial temperature, T₁ = 9.00 °CFinal temperature, T₂ = 45.0 °CChange in temperature, ΔT = 36 °CMass, M = 3.40 gHow do I determine the mass of the iron?The following data were obtained from the question:
Heat energy absorbed (Q) = 55.0 JSpecific heat capacity of iron (C) = 0.449 J/gºC Initial temperature of water (T₁) = 9.00 °CFinal temperature of water (T₂) = 45.0 °CChange in temperature (ΔT) = 45 - 9 = 36 °CMass of iron (M) =?The mass of the iron can be obtain as shown below:
Q = MCΔT
Inputting the given parameters, we have
55 = M × 0.449 × 36
55 = M × 16.164
Divide both sides by 16.164
M = 55 / 16.164
M = 3.40 g
Thus, we can conclude from the above calculation that the mass of the iron is 3.40 g
Learn more about mass:
https://brainly.com/question/1674804
#SPJ1
describe the experimental method through which you determine the appropriate wavelength for an analyte
Answers
Wavelength is chosen by analyzing the spectrogram.
Wavelength can be defined as the distance between two successive crests or troughs of a wave. It is measured in the direction of the wave.
A spectrogram gives a going for walks show of a sound signal because it takes place in real time, a spectrum, however, offers us a photograph of the sound at a particular factor in time. A spectrum can permit you to see.
Spectrograms map out sound in a comparable way to a musical rating, only mapping frequency rather than musical notes. Seeing frequency energy allotted over the years in this manner allows us to genuinely distinguish each of the sound factors in a recording, and their harmonic shape.
Learn more about wavelength here:- https://brainly.com/question/10728818
#SPJ4
emergency (30 points) what conclusion can you make about the relationship between temperature and pressure?
Answers
Answer:
This relationship between pressure and volume is known as Boyle's lawA law that states that at constant temperature, the volume of a fixed amount of a gas is inversely proportional to its pressure., after its discoverer, and can be stated as follows: At constant temperature, the volume of a fixed amount of a gas is. The pressure law states that for a constant volume of gas in a sealed container the temperature of the gas is directly proportional to its pressure. This can be easily understood by visualising the particles of gas in the container moving with a greater energy when the temperature is increased.
Explanation:
Answer:
basically increased temperature causes an increase in pressure. and vise versa.
What is the compound name of BaSO4?
What is the molar mass?
What is the mass in grams of 2.3 mols of the compound
Answers
The inorganic substance with the chemical formula BaSO4 is barium sulfate (or sulphate). It is a tasteless, crystalline white substance that is insoluble in water.
BaSO4 stands for barium sulfate.A barium cation and a sulfate anion are the two elements that make up barium sulfate. There are four oxygen atoms joined to the sulfur. A sulfate salt of barium, known as BaSO4, is present in the mineral barite. It is a white crystalline substance that is soluble in strong acids but insoluble in water and alcohol.
Is barium the same as bromine?Elements include both barium and bromine. Barium is a metal belonging to Group 2 of the modern periodic table, and bromine is a non-metal belonging to Group 17 (halogen).
To know more about chemical formula visit:-
https://brainly.com/question/29031056
#SPJ1
Glucose (C6H12O6) and altrose (C6H12O6) can form glycosidic bonds to create polysaccharides. What is the chemical formula of a polymer made from 4 glucose and 2 altrose
Answers
Polysaccharides are formed by bonding monosaccharides through a glycosidic bond. This is what allows us to obtain long chains of monosaccharides that form the structural support for the cell walls of plants and animals. To determine the chemical formula of a polymer made from 4 glucose and 2 altroses,
we will first write down the molecular formula of each monosaccharide. Glucose: C6H12O6Altrose: C6H12O6A polymer of 4 glucose monosaccharides would have the chemical formula: 4(C6H12O6) = C24H42O21A polymer of 2 altrose monosaccharides would have the chemical formula: 2(C6H12O6) = C12H22O11To determine the chemical formula of a polymer made from 4 glucose and 2 altroses,
we need to add the two chemical formulas together: C24H42O21 + C12H22O11 = C36H64O32Therefore, the chemical formula of a polymer made from 4 glucose and 2 altroses is C36H64O32.
to know more about Glucose here:
brainly.com/question/13555266
#SPJ11
how much energy is required to decompose 765g of pcl3
Answers
The amount of energy required to decompose 765g of PCl₃ is 887.7 kJ calculated by using the formula: Q = m × ∆H.
To calculate the amount of energy required to decompose 765g of PCl₃, we need to find the enthalpy change (∆H) of the reaction. According to the balanced equation, 1 mole of PCl₃ decomposes to form 1 mole of PCl₅ and 1 mole of Cl₂. The enthalpy change for this reaction can be found using Hess's Law or from the enthalpy of formation values of the reactants and products.
The enthalpy change of the reaction is ∆H = ∆Hf(PCl₅) + ∆Hf(Cl₂) - ∆Hf(PCl₃)
Substituting the values, we get: ∆H = (-128.2) + (0) - (-287.5) = 159.3 kJ/mol
Now, we can use the formula Q = m × ∆H to calculate the amount of energy required to decompose 765g of PCl₃.
Number of moles of PCl₃ = 765/137.33 = 5.57 mol
Amount of energy required = 5.57 mol × 159.3 kJ/mol = 887.7 kJ
Therefore, the amount of energy required to decompose 765g of PCl₃ is 887.7 kJ.
Learn more about Hess's Law here:
https://brainly.com/question/10504932
#SPJ11
✓ Details
1. 2HCl(aq) + Zn(s)→ H₂(g) + ZnCl₂(aq)
You have 100. mL of a .300 M solution of HCI. As the reaction occurs, the volume of solution is unchanged.
1. How many grams of Zn were used up?
2. How many liters of hydrogen gas at STP were formed?
3. What is the molar concentration of the zinc chloride formed?
k

Answers
The number of grams of Zinc used is 1962g, the volume of hydrogen gas formed at STP is 672.071L and the molar concentration of Zinc Chloride is 300M.
1. The number of moles of Zn used up is given by the equation:
n = CV
Since the volume is given in mL, we must convert it to L before plugging it into the equation.
n = (300 mol/L)(100 mL)(1 L/1000 mL)
n = 30 mol
The molar mass of Zn is 65.39 g/mol, so the number of grams of Zn used up is given by:
m = nM
m = (30 mol)(65.39 g/mol)
m = 1962 g
2. The number of moles of H₂ formed is also 30 mol. One mole of any gas occupies 22.4 L at STP, so the number of liters of H₂ formed is given by:
V = nRT/P
V = (30mol)(0.08206 L•atm/mol•K)(273 K)/(1 atm)
V = 672.071 L
The volume of liters of H2 formed is 0.6L
3. The molar concentration of ZnCl₂ is given by the equation:
C = n/V
Here, number of moles is n and the volume is V The number of moles of ZnCl₂ is 30 mol, and the volume is 100 mL, so the molar concentration is:
C = (30 mol)/(0.1L)
C = 300 M
To know more about normality, click below:
https://brainly.com/question/9754178
#SPJ1
these ice caves only form when temperatures are freezing. at what temperature does water freeze?
Answers
Answer:
water freezes at 32 degrees Fahrenheit (0 degrees Celsius)
Explanation:
determine how much heat (in kj) of 2.89 mol of tio2(s)
Answers
Total heat generated by 2 mole of TiO2(s) is 4.963kJ.
The amount of heat released in the reaction of 2.89 mol of TiO2(s) can be calculated using the following equation: q = nCΔT, where n is the number of moles, C is the specific heat capacity of TiO2, and ΔT is the change in temperature.
The specific heat capacity of TiO2 is 683. 697. J/kgK. and the change in temperature is is 25k. By plugging in the values and converting J to kJ,
q = 2.89 * 25 * 683.697
=> 4963.35
In brief, the amount of heat released by 2.89 mol of TiO2(s) is 4.963kJ.
To know more about specific heat capacity click on below link:
https://brainly.com/question/16952828#
#SPJ11
Complete question :
determine how much heat (in kj) of 2.89 mol of tio2(s) with a temperature difference of 25k
be fast i need the answer fast.

Answers
d is the answer. according to me
The reactants in a chemical reaction are shown below.
Li₂CO3 + H2SO4
Answers
Answer:
Li2SO4+H2CO3
Explanation:
put d equation this way
negative ions to positive ions
Express 36,000,000 in scientific notation
Answers
Answer:
\(3.6 * 10^{7}\)
Explanation:
Hope this helps!
Rank the following in order of decreasing the rate of effusion. F2 SF6 He Ar a. He > F2 > SF6 > Ar b. He > F2 > Ar > SF6 c. Ar > He > SF6 > F2 d. SF6 > Ar > F2 > He e. OF2 > Ar> He > SF6
Answers
He> F2 > Ar > SF6.
How may effusion rates be compared?A square root of a gas's molecular weight has an inverse relationship with the rate for effusion of that gas.A gas will effuse faster when it is lighter and more slowly when it is heavier.
N2 or O2: which has a greater effusion rate?In comparison to oxygen, nitrogen has a smaller molar mass.As a result, the rate of nitrogen effusion is greater than that of oxygen effusion.
To know more about order visit:
https://brainly.com/question/13467963
SPJ4
Label each variable in the equation below with the property it represents. number of moles time power voltage volume molarity Nsolute RT = MRT rate universal gas constant soln osmotic pressure absolute temperature integer molar mass
Answers
There are 16 variable in the equation below with the property it represents. number of moles time power voltage volume molarity .
"number of moles" represents n (the number of moles of a substance)
"time" represents t (time elapsed)
"power" represents P (power, or the rate at which energy is transferred)
"voltage" represents V (potential difference, or voltage)
"volume" represents V (volume)
"molarity" represents M (molarity, or the concentration of a solution)
"N solute" represents N (the number of solute particles in a solution)
"RT" represents RT (the product of the gas constant R and the absolute temperature T)
"MRT" represents MRT (the product of moles (n), the gas constant (R), and absolute temperature (T))
"rate" represents rate (the rate at which a reaction occurs)
"universal gas constant" represents R (the universal gas constant, a physical constant used in equations of state)
"sol" represents solution (a shortened form of "solution")
"osmotic pressure" represents π (osmotic pressure, the pressure that needs to be applied to a solution to stop osmosis)
"absolute temperature" represents T (absolute temperature, temperature measured on the Kelvin scale)
"integer" represents an integer value
"molar mass" represents M (molar mass, the mass of one mole of a substance)
Learn more about moles:
brainly.com/question/26416088
#SPJ4
Label each variable in the equation below with the property which represents. number of moles time power voltage volume molarity N solute RT = MRT rate universal gas constant solution osmotic pressure absolute temperature integer molar mass.
What is the volume of 5.07 grams of copper? The density of copper is 8.96g/mL
Answers
Explanation
Given:
Mass of copper = 5.07 g
Density of copper = 8.96 g/mL = 8960 g/L
Requested: Volume of copper
Solution
p = m/V where p is the density, m is the mass and V is the volume
m = p x V
V = m/p
V = 5.07 g/8.96 g/mL
V = 0.566 mL
Answer
Volume of copper = 0.566 mL
If your reaction between benzophenone and sodium borohydride
asked you to quench with acid (HCl). What precautions should you
take before adding the acid?
Check all that apply
-You should add a boiling stone before the acid is added.
-The acid should be added dropwise.
-You should add a base before adding the acid to make sure the
solution is neutral when you add the HCl.
-The reaction solution should be cooled to room temperature.
-You should add the acid as quickly as possible to ensure
quenching is complete.
Answers
When quenching with acid (HCl) after a reaction between benzophenone and sodium borohydride, the following precautions should be taken: The acid should be added dropwise, the reaction solution should be cooled to room temperature, and a base should be added before adding the acid to make sure the solution is neutral. Therefore, options b, c and d are the correct answers.
In chemistry, quenching refers to the cooling of a hot object in a liquid to stabilize the shape and other physical properties of the object. This process involves cooling a substance down to a level where it can be manipulated without changing shape or other physical characteristics.
However, when quenching with acid, the objective is different. Acid quenching is used to stop chemical reactions or remove unreacted chemicals after a chemical reaction has taken place. It helps to minimize side reactions and/or remove excess reagents.
To learn more about acid, click here:
https://brainly.com/question/14072179
#SPJ11
If anyone is good at chemistry do you mind helping? (●'◡'●)

Answers
• Before the balloon was placed inside the hot water, the pressure was the same inside and outside the balloon. The hot water raised the kinetic energy of the air molecules inside the balloon, expanding the balloon, through thermal expansion.
• (1) the pressure of air inside the balloon increased, (2) the volume of the inside of the balloon increased as well, and (3) the temperature of the balloon increased. Note that pressure and volume are inversely proportional, and pressure and temperature are directly proportional. Therefore as the temperature increases, the pressure inside will increase, causing an increase in the volume. At a certain point though the volume will increase too much as to cause a significant decrease in pressure.
• The air molecules will gain kinetic energy, hence (1) increasing the molecules's speed, and (2) heating the air molecules.
Williamson synthesis of 1-isopropoxy-1-methylcyclopentane. O Williamson ether synthesis would give a poor yield of product as the product does not have Markovnikov orientation.O Williamson ether synthesis would give a poor yield of product as the product does not have anti-Markovnikov orientation. O Williamson ether synthesis would give a poor yield of product as the halide is on a 3º carbon.O Williamson ether synthesis would give a poor yield of product as the halide is on a 2° carbon.
Answers
Williamson ether synthesis would give a poor yield of product as the halide is on a 2° carbon. is true about the synthesis of 1-isopropoxy-1-methylcyclopentane.
The Williamson ether synthesis is a method for the synthesis of ethers using an alcohol and an alkyl halide. The reaction proceeds through a nucleophilic substitution mechanism and the ether product is obtained with the alcohol and halide groups in the anti-Markovnikov orientation. The reactivity of the alkyl halides used in this reaction follows the order: primary > secondary >> tertiary. Therefore, the reaction of a secondary halide such as 2° carbon halide will give a poor yield of product as the reactivity of 2° carbon halide is less compared to primary halide. As a result, the reaction is less efficient and the yield of the product is lower.
To know more about Williamson ether synthesis click below:
https://brainly.com/question/29434473#
#SPJ4
2.91 moles of aluminum are how many grams(with work)
Answers
Which of the following types of energy is question 4 of 5
associated with movement?
O Potential
O Gravitahonal
O Kinetic
O Chemical
Answers
Answer:
Explanation:
Which statement(s) best describe why table sugar is
considered a pure substance?
SELECT ALL THAT APPLY
a Sugar is a mixture of pure compounds
d
b A bowl of sugar contains only one compound
c Sugar is solid like all other pure substances
Sugar cannot be separated further by physical
means
Sugar has the same chemical composition
e throughout
Answers
Best describe table sugar is considered a pure substance is Sugar has the same chemical composition throughout
Table sugar is pure sucrose derived from sugar beet or sugar cane and sucrose is the disaccharide consisting of glucose and fructose and it is produced by green plant in the process of photosynthesis and since the chemical composition of sugar is definite and does not vary hence it is pure substance and table sugar refer to standard while white sugar that you see in your cooking baking or cup of tea at home and the scientific name foe table sugar is sucrose
Know more about pure substance
https://brainly.com/question/143254
#SPJ1
Which set of numbers will balance the following equations? 1's have been included for clarity.__Mn3N4 + __NaF --> __MnF4 + __Na3N a 1; 4; 1; 4 b 1; 4; 3; 2 c 1; 12; 3; 4 d 3; 2; 3; 2
Answers
ANSWER
Option C
EXPLANATION
Given that;
\(\text{ ----- Mn}_3N_4\text{ }+\text{ ---- NaF }\rightarrow\text{ ---- MnF}_4\text{ }+\text{ ---Na}_3N\)In the reaction above, we have the following data
At the reactants side;
3 atoms of manganese
4 atoms of nitrogen
1 atom of sodium
1 atom of fluorine
At the products side
1 atom of manganese
4 atoms of fluorine
3 atoms of sodium
1 atom of nitrogen
To balance the above equation, apply the law of conservation mass
Law of conservation of mass states that matter can neither be created nor destroyed but can e transformed from one formato another.
To balance the equation, 1 mole of Mn3N4 reacts with 12 moles of Na Tto give 3 moles of MnF4 and 4 moles of Na3N
So, the new equation becomes
\(\text{ Mn}_3N_4\text{ }+\text{ 12NaF }\rightarrow\text{ 3MnF}_4\text{ }+\text{ 4Na}_3N\)The following data can be deduced in the above equation
At the reactants side
3 atoms of Mn
4 atoms of N
12 atoms of Na
12 atoms of F
At the products side
3 atoms of Mn
12 atoms of F
12 atoms of Na
4 atoms of N
Looking atthe vabove data, the number of atoms of each element at the reactants side is equal to the number of atoms of same elements at the products side.
Hence, the correct answer is option Ce
u
Compare and contrast control group and experimental group.
Answers
Answer: An experimental group, also known as a treatment group, receives the treatment whose effect researchers wish to study, whereas a control group does not
Explanation:
The control group and experimental group are compared against each other in an experiment. The only difference between the two groups is that the independent variable is changed in the experimental group. The independent variable is "controlled" or held constant in the control group
Numbering the Steps for Balancing Equations Number the steps for balancing equations: Use coefficients to increase the atoms on each side. Check to make sure you have the same number of each type of atom on each side. Count the atoms on each side. Identify the atoms on each side. Intro Done
Answers
In order to balance a chemical equation steps to be followed are : Identify atoms on each side.Count atoms on each side.Numbering steps for balancing Equations in sequential order.Use coefficients to increase atoms on each side.Check to make sure you have same number of each type of atom on each side.
What is chemical equation?Chemical equation is a symbolic representation of a chemical reaction which is written in the form of symbols and chemical formulas.The reactants are present on the left hand side while the products are present on the right hand side.
A plus sign is present between reactants and products if they are more than one in any case and an arrow is present pointing towards the product side which indicates the direction of the reaction .There are coefficients present next to the chemical symbols and formulas .
Learn more about chemical equation,here:
https://brainly.com/question/30087623
#SPJ9
Your question is is incomplete, the complete question will be:
Numbering the Steps for Balancing Equations in a sequential order:
Use coefficients to increase the atoms on each side.
Check to make sure you have the same number of each type of atom on each side.
Count the atoms on each side.
Identify the atoms on each side.
when earth is closest to the sun, the northern hemisphere is in winter, why is this true?
Answers
Explanation:
Opposite hemispheres have opposite seasons, no matter the earth's rotation, because of the earth's tilt. The earth's tilt is responsible for all seasons.
2 HCl (9)
=H2(g) + Cl2 (9)
K = [H][CI]/[HCI)
UK = [H][CI]/2[HCI]
K = 2[HC]/[H]CI.)
K = [HCI)?/[H][1]
Answers
Answer:
(K) = [H2] [Cl2] / [HCl]²
Explanation:
The equation of the reaction is given as;
2 HCl (g) ⇔ H2 (g) + Cl2 (g)
The Equilibrium constant is the ratio of the concentration of the products and reactants raised to the power of their coefficients
Products = H2 and Cl2
Reactants = HCl
Equilibrium constant (K) = [H2] [Cl2] / [HCl]²
Sample of calcium carbonate [CaCO3 (s)] absorbs 45. 5 J of heat, upon which the temperature of the sample increases from 21. 1 °C to 28. 5 °C. If the specific heat of calcium carbonate is 0. 82 J/g-K, what is the mass (in grams) of the sample?
A. 3. 7
B. 5. 0
C. 7. 5
D. 410
E. 5. 0 x 103
Answers
The mass of the sample of calcium carbonate when it absorbs 45. 5 J of heat is option B: 5.0 grams.
This is a heat transfer problem The general formula for heat transfer or heat change (as in absorption) is as follows:
Q = m x c x ΔT
where:
Q = heat absorbed,
m = mass of the substance,
c = specific heat, and
ΔT = change in temperature.
We are already given:
Q = 45.5 J
ΔT is the difference between 28.5 °C and 21.1 °C:
28.5 °C - 21.1 °C = 7.4 °C
c = 0.82 J/g-K
We need to find the mass of the sample or the substance:
m = Q/ c x ΔT
= 45.5 / 0.82 x 7.4
= 5.0 grams
To know more about heat transfer, refer:
https://brainly.com/question/25898569
#SPJ4
100 points
A sample of an element was poured from one container into a second container. It took on the shape of the second container. It was then cooled. After cooling, the element was removed from the container and kept its new shape. What happened to the atoms of the element when they were cooled in the second container?
A) They began to move more quickly.
B) They began to escape into the air.
C) They stopped moving past the atoms around them.
D) They stopped being attracted to nearby atoms.
Answers
Answer:
C
Explanation:
When the atoms were cooked they became a solid, or kept being a liquid so the shape of the element would of not changed.
asap! first to answer correctly gets 10 pts plus brainliest! :)

Answers
Answer:
c
Explanation:
c first quarter waxing half moon
Which of the following statements on HPLC modes is true? A. Increasing the polarity of the mobile phase decreases the elution time of polar compounds in normal-phase HPLC B. A non-polar stationary phase is used in normal-phase HPLC C. Compounds have a lower attraction to the mobile phase than to the stationary phase in displacement development D. A polar stationary phase is used in reversed-phase HPLC E. More polar compounds elute first in normal-phase HPLC
Answers
The following statements on HPLC modes are true is more polar compounds elute first in normal-phase HPLC (Option E).
The liquid chromatography (HPLC) is a technique in analytical chemistry employed for the separation, identification, and quantification of elements. It is considered a highly sensitive method, and it works by separating the components in a mixture with the assistance of a solvent under high pressure.
There are two modes of HPLC: Reversed-Phase HPLC (RP-HPLC) and Normal-Phase HPLC (NP-HPLC). In RP-HPLC, a nonpolar stationary phase, such as C18, is used, and polar solvents, such as water, are used as mobile phases. Polar stationary phases, such as silica gel, are used in NP-HPLC, while nonpolar solvents, such as hexane, are used as mobile phases.
More polar compounds have a greater affinity for the polar stationary phase than less polar compounds, which have a higher affinity for the nonpolar mobile phase in NP-HPLC. As a result, less polar compounds elute first in normal-phase HPLC.
Thus, the correct option is E.
Learn more about HPLC: https://brainly.com/question/13490391
#SPJ11