A 25.0 mL volume of HCl solution of unknown concentration is titrated with a 0.148 M NaOH solution. Neutralization of the HCl solution requires 39.1 mL of the NaOH solution. Calculate the concentration of the HCl solution.
Answers
The concentration of the HCl solution is 0.231 M.
To calculate the concentration of the HCl solution, we can use the concept of stoichiometry.
The balanced chemical equation for the reaction between HCl and NaOH is:
HCl + NaOH → NaCl + H2O
From the equation, we can see that 1 mole of HCl reacts with 1 mole of NaOH.
First, let's calculate the number of moles of NaOH used in the titration:
moles of NaOH = concentration of NaOH solution * volume of NaOH solution used
= 0.148 M * 39.1 mL
= 0.0057818 mol
Since the stoichiometry of the reaction is 1:1, the number of moles of HCl in the solution is also 0.0057818 mol.
Next, we can calculate the concentration of the HCl solution:
concentration of HCl solution = moles of HCl / volume of HCl solution
= 0.0057818 mol / 25.0 mL
= 0.231 M
Therefore, the concentration of the HCl solution is 0.231 M.
For such more questions on concentration
https://brainly.com/question/30677144
#SPJ11
Related Questions
Which of these describes why a stent is used?
O A. To open an artery near the heart
O B. To repair a weak heart valve
O C. To increase the biocompatibility of a heart transplant
O D. To enhance the durability of a pacemaker
SUBMIT
Answers
Answer:the correct answer is A.To open an artery near the heart
Explanation:I just took the test :)
A stent is used to open an artery near the heart and the correct option is option A.
A stent is commonly used in medical procedures to open up narrowed or blocked arteries near the heart. It is a small, mesh-like tube typically made of metal or synthetic materials. The stent is inserted into the affected artery and expands, effectively widening the artery and improving blood flow. This procedure is known as coronary angioplasty or stenting.
The narrowing or blockage of arteries can occur due to atherosclerosis, a condition in which plaque builds up on the artery walls. This can lead to reduced blood flow and potentially result in chest pain (angina) or even a heart attack. By placing a stent, the artery is opened up, allowing for improved blood flow to the heart muscle.
Thus, the ideal selection is option A.
Learn more about Stent, here:
https://brainly.com/question/30477421
#SPJ6
Which statements are true of the reaction below? 2Na(s) + Cl2(g) ---> 2NaCl(s)

A.
Na(s) is a reactant.

B.
NaCl is a product.

C.
NaCl is a liquid.

D.
Cl2 is a solid.
Answers
Answer:
A and B are both true
Explanation:
Na is REACTING with Cl2 to PRODUCE NaCl
How many moles of O2 would there be if I had 4 moles of Fe
Answers
4 moles of Fe react with 3 moles of oxygen. Therefore, the mole ratio of iron to iron oxide in this process is 4:2.
What is oxygen ?The chemical element with the atomic number 8 and symbol O is called oxygen. It belongs to the periodic table's halogen group, is a very reactive nonmetal, and an oxidizing agent that easily produces oxides with most elements as well as other compounds.
The non-metallic element oxygen occurs naturally as a molecule. Two oxygen atoms that are tightly bound together make up each molecule. Oxygen is in a gaseous form at ambient temperature due to its low melting and boiling temperatures.
According to scientists, the oceans produce between 50 and 80 percent of the oxygen used on Earth. Oceanic plankton, which includes floating plants, algae, and certain bacteria that can photosynthesize, is the main source of this production.
Thus, 4 moles of Fe react with 3 moles of oxygen.
To learn more about oxygen, follow the link;
https://brainly.com/question/1506082
#SPJ1
What Element am I?
I have 6 Valence Electrons.
I am in the third row.
My atomic mass is less than Selenium , but it is more than Oxygen. I need the answer ASAP
Answers
Answer:
The correct answer is - sulfur.
Explanation:
In the periodic table, there are 18 groups and 7 rows or periods arranged according to their atomic number or electronic configuration. In the question, it is mentioned that the desired element atomic mass is less than the atomic mass of the selenium which is 78.96, and more than oxygen which is 15.99 with 6 electron valence and present in the third row.
As it has 6 valency of electron it must be in the 16 group of the table that comprises the 6 valency and as it is located in the 3rd row it must be sulfur that also has an atomic mass between selenium and oxygen.
where does energy come from? newsela
Answers
Answer:
All of our energy comes from the sun.
Explanation:
The sun sends out huge amounts of energy through its rays every day. Without the sun, life on earth would not exist, since our planet would be totally frozen.
how many atoms are there in 6.2 grams of silver
Answers
Perform the following
mathematical operation, and
report the answer to the
appropriate number of
significant figures.
15.67 - 6.943 = [?]
Answers
Answer:
8.73
Explanation:
when you are adding or subtracting numbers, the sigfig (significant figure) is based on how many numbers after the decimal. with this info, we can see that 15.67 has 2 sigfigs and 6.943 has 3 sigfigs. when you subtract normally, you would get 8.727, which has 3 sigfigs, so you would round the last 7 up to get 8.73 with 2 sigfigs!
also it is to 2 sigfigs because we know that we go by the least number of sigfigs. hope this helped!
what is the main material that make up the tusk of an elephant
Answers
Answer:
Ivory
Explanation:
The outside of the tusk is made of ivory, but 2/3 of the tusk is their head, made of tissue. But I think the answers Ivory. Thats the reason why poachers go for the tusks. Because humans like ivory :/
As a result of this process, the proportions of oxygen and carbon dioxide in
air breathed in and air breathed out change.
Which one of the statements is true? Tick the correct box. [1]
- Air breathed out has less carbon dioxide and more oxygen than air breathed in.
- Air breathed out has less carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and more oxygen than air breathed in.
Answers
Answer:
the third one
Explanation:
When you breathe in, you inhale oxygen and exhale carbon dioxide
What is a term of a minority component of a solution
Answers
Answer:
the minority component of a solution, the "dissolved" aqueous solution. water is the solvent, and a solid, liquid, or gas is the solute.
Explanation:
Burning of paper is chemical change why? write with reaction.
Answers
Answer:
Burning of paper is not a physical change.It is chemical change as ash is formed in the process which is new compound and oxides of carbon are also released during the process!
can someone help me solve the questions below using the data table below PLEASEE
DATA TABLE:
Mass of flask and vinegar solution- 25.17g
Mass of flask- 15.12g
Volume of vinegar solution (in mL)- 10.00ml
Initial volume of NaOH (in mL)-0.00ml
Final volume of NaOH (in mL)-39.00ml
CALCULATIONS:
Mass of vinegar solution- 10.0503g
Volume of NaOH used in titration (in mL)-39.00ml

Answers
The molarity of NaOH solution is 0.114 M.
Given, Mass of flask and vinegar solution= 25.17 g.Mass of flask= 15.12 gVolume of vinegar solution (in mL)= 10.00 mlInitial volume of NaOH (in mL)= 0.00 mlFinal volume of NaOH (in mL)= 39.00 mlThe Mass of vinegar solution is 10.0503 g.The volume of NaOH used in titration is 39.00 ml.Let's calculate the molarity of the NaOH solution.First, calculate the moles of NaOH used in the reaction. Moles of NaOH = Molarity × Volume of NaOH (in L) Converting volume in mL to L,Volume of NaOH used = 39.00 mL = 39.00/1000 L = 0.0390 LThe molarity of NaOH solution is given by;Molarity of NaOH = Moles of NaOH / Volume of vinegar solution (in L)Converting volume in mL to L,Volume of vinegar solution = 10.00 mL = 10.00/1000 L = 0.0100 LNow, substituting the values; Molarity of NaOH = 0.114 M.
for such more questions on molarity
https://brainly.com/question/30404105
#SPJ8
What is the net ionic equation for the reaction shown below?
AgNO3(aq) + NaCl(aq) --> AgCl(s) + NaNO3(aq)
Answers
Answer:
Ag⁺(aq) + Cl⁻(aq) → AgCl(s)
Explanation:
Chemical equation:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
Balance chemical equation:
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
Ionic equation
Ag⁺(aq)+ NO₃⁻(aq) + Na⁺(aq)+ Cl⁻(aq) → AgCl(s) + Na⁺(aq)+ NO₃⁻(aq)
Net ionic equation:
Ag⁺(aq) + Cl⁻(aq) → AgCl(s)
The NO₃⁻((aq) and Na⁺ (aq) are spectator ions that's why these are not written in net ionic equation. The AgCl can not be splitted into ions because it is present in solid form.
Spectator ions:
These ions are same in both side of chemical reaction. These ions are cancel out. Their presence can not effect the equilibrium of reaction that's why these ions are omitted in net ionic equation.
Read this excerpt from Narrative of Sojourner Truth What type of external conflict does it describe?
When her master saw her, he said, Well, Bell, so you ve run away from me.' No, I did not run away, I walked away by day-night, and all
because you had promised me a year of my time. His reply was, You must go back with me. Her decisive answer was, No, I won't go back
with you.' He said, Well, I shall take the child. This also was stoutly negatived.
OA person versus person
ОВ.
person versus nature
Ос
person versus society
OD
person versus self
Answers
Answer:
Person versus person conflict.
Explanation:
Convert 520 inches to cm using the conversion factor that 1 inch = 2.54 cm.
1320 c
O 45.59 cm
O 0.005 cm
O205 inches
Answers
Answer:
Explanation:
520:2.54=204.7 round to 205 the answer=205 cm=520 inches
In the measurement 0.342, which number is the estimated digit?
Answers
Answer:
"The last digit in any number is referred to as the estimated digit."
Explanation:
So it should be 2
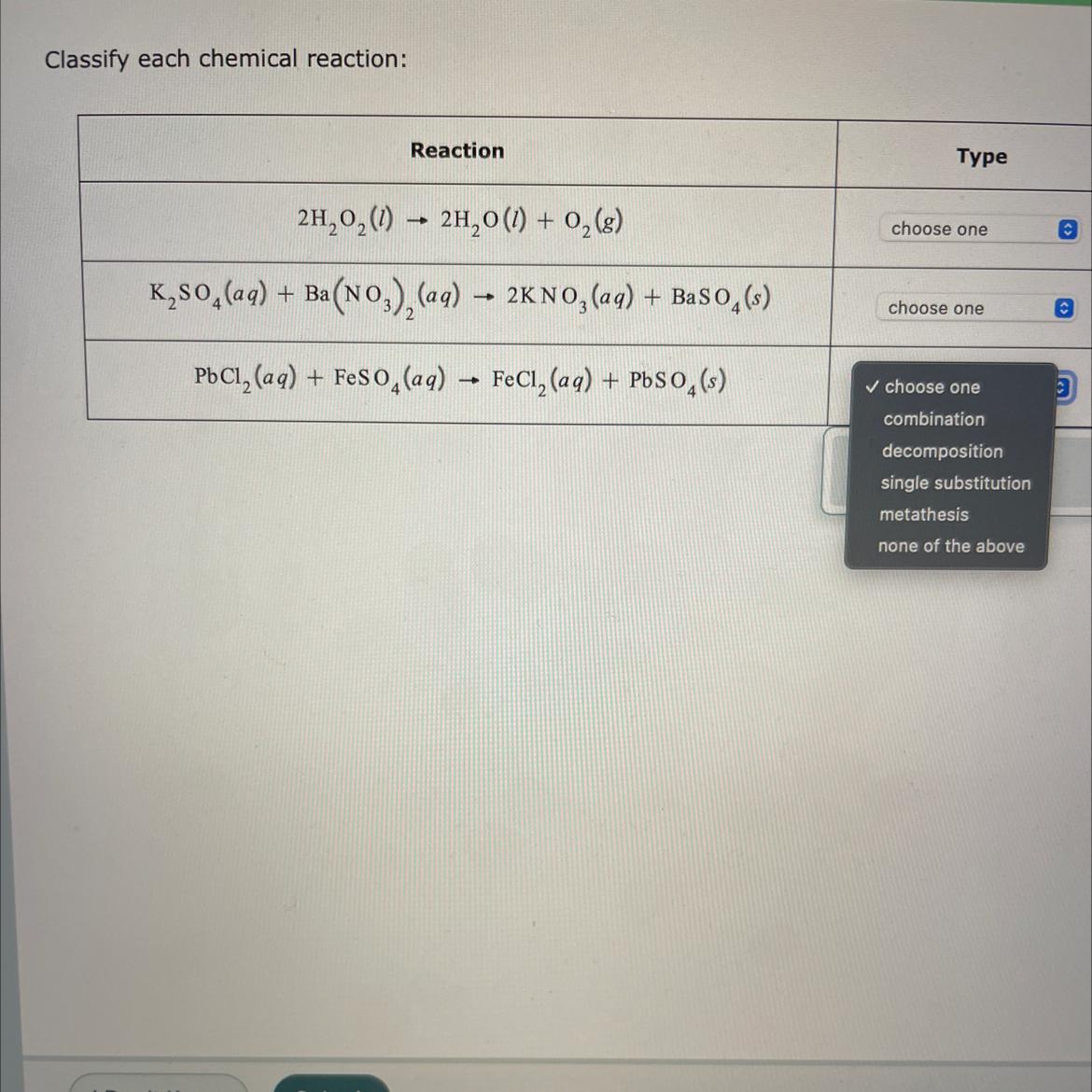
Classify each chemical reaction:Reaction2H₂O₂(1)→ 2H₂O(1) + 0₂ (8)K₂SO4 (aq) + Ba(NO3)₂(aq) → 2KNO3(aq) + BaSO4(s)PbCl₂ (aq) + FeSO4 (aq) → FeCl₂ (aq) + PbSO4(s)Typechoose onechoose one✓ choose onecombinationdecompositionsingle substitutionmetathesisnone of the aboveO
Answers
A decomposition reaction is a chemical reaction in which one reactant breaks down into two or more products.
A metathesis reaction is a chemical reaction in which the positive ions and negative ions present in the reactants appear to exchange partners.
6. A diamond contains 5.0 ×10^21 atoms of carbon. What amount(moles) of carbon and what mass (grams) of carbon are in this diamond? *
a. 0.008303 and 0.0997
b. 0.008303g and 0.0997mol
c. 0.008303amu and 0.0997
d. 0.008303 and 0.0997amu
Answers
Answer:
0.008303 mol and 0.0997 g
Explanation:
From Avogadro's hypothesis, we understood that 1 mole of any substance contains 6.022×10²³ atoms.
This implies that 1 mole of carbon also contains 6.022×10²³ atoms.
Next, we shall determine the mole of carbon that contains 5.0×10²¹ atoms. This can be obtained as follow:
1 mole of carbon contains 6.022×10²³ atoms.
Therefore, Xmol of carbon will contain 5.0×10²¹ atoms i.e
Xmol of carbon = 5.0×10²¹ / 6.022×10²³
Xmol of carbon = 0.008303 mole
Therefore, 0.008303 mole of carbon contains 5.0×10²¹ atoms.
Finally, we shall determine the mass of carbon. This can be obtained as follow:
Mole of carbon = 0.008303 mole
Molar mass of carbon = 12.01 g/mol
Mass of Carbon =.?
Mole = mass /Molar mass
0.008303 = mass of carbon/12.01
Cross multiply
Mass of Carbon = 0.008303 x 12.01
Mass of Carbon = 0.0997 g.
who like math ? Cause I don’t.
Answers
Answer:
heheheheheh... I like it
A football player runs 80 yards for a touchdown! It takes him 20 seconds. What is his speed?

Answers
Answer:
4 yards/second is the answer
Answer:
the answer is 4 yards per second
Explanation:
80 yards in 20 seconds
20+20+20+20=80
there are 4, 20s to make 80
in other words...
80÷4=20
Which of the following solutions will be expected to have the highest vapor pressure? 0.10 m Al(CIO) 0.50 m Ca(CIO4)2 O 0.30 m Naci 0.75 m C,H,OH 0.10 m KCIO
Answers
The solution with the highest vapor pressure will be the one with the lowest boiling point and the most volatile components.
According to Raoult's law, the vapor pressure of a solution is directly proportional to the mole fraction of the solvent. So, in this case, the solution with the lowest amount of solute would have the highest vapor pressure. From the options given, the solution with the lowest concentration of solute is 0.75 m C2H5OH. Ethanol has a lower boiling point and is more volatile compared to the other solutes, thus the solution with 0.75 m C2H5OH is expected to have the highest vapor pressure.
To determine the solution with the highest vapor pressure, we need to consider Raoult's law, which states that the vapor pressure of a solution is directly proportional to the mole fraction of the solvent. In this case, the solution with the lowest molality will have the highest vapor pressure since it has the highest mole fraction of the solvent. Among the given solutions, 0.10 m Al(ClO) has the lowest molality, making it the solution expected to have the highest vapor pressure.
To know about vapor :
https://brainly.com/question/30820393
#SPJ11
What is the wave length of the longitudinal wave of frequency 20khz which propagates at 360m/s
Answers
The wavelength of a longitudinal wave can be calculated using the formula λ = v/f. the wavelength of the longitudinal wave with a frequency of 20 kHz is approximately 18 millimeters.
To calculate the wavelength (λ) of a longitudinal wave, we use the formula λ = v/f, where
v represents the velocity of the wave and
f is the frequency.
In this case, the longitudinal wave has a frequency of 20 kHz, which can be converted to 20,000 Hz. The velocity of the wave is given as 360 m/s.
Substituting these values into the formula, we have:
λ = 360 m/s / 20,000 Hz
Simplifying the calculation, we find:
λ = 0.018 m or 18 mm
Therefore, the wavelength of the longitudinal wave with a frequency of 20 kHz and propagating at 360 m/s is approximately 0.018 meters or 18 millimeters.
To know more about frequency , click here-
brainly.com/question/28995449
#SPJ11
9. a pot of water is placed on a burner on a gas stove to heat. which part is the source of the activation energy required (the flame/the water), and is this reaction endothermic or exothermic?
Answers
The part that is the source of the activation energy required is the flame and the reaction is endothermic.
Endothermic reactionsIn this scenario, the source of the activation energy required is the flame on the gas stove. The flame provides the heat energy necessary to increase the kinetic energy of the water molecules and cause them to move faster, resulting in an increase in the temperature of the water.
The process of heating the water is an endothermic reaction because energy is being absorbed from the surroundings (in this case, from the flame) in order to increase the temperature of the water.
As the water absorbs heat energy from the flame, the potential energy of the water molecules increases, causing them to move faster and collide more frequently, which leads to an increase in the temperature of the water.
More on endothermic reactions can be found here: https://brainly.com/question/23184814
#SPJ1
What happens in cellular respiration?

Answers
Answer:
During aerobic cellular respiration, glucose reacts with oxygen, forming ATP that can be used by the cell. Carbon dioxide and water are created as byproducts. In cellular respiration, glucose and oxygen react to form ATP. Water and carbon dioxide are released as byproducts.
Explanation:

An object must be immersed in quaternary ammonium for at least how many seconds?
Answers
An object must be immersed in quaternary ammonium for at least 30 seconds to be effectively disinfected. This is according to the CDC guidelines for chemical disinfectants.
The required duration for an object to be immersed in quaternary ammonium solution depends on the specific product being used and the intended purpose.
The manufacturer's instructions should always be followed for any disinfectant or cleaning agent, including quaternary ammonium solutions. In general, the contact time recommended by the manufacturer should be followed, which typically ranges from 30 seconds to several minutes.
It is important to note that if the recommended contact time is not followed, the disinfectant may not be effective in killing bacteria, viruses, or other pathogens. Therefore, it is important to carefully read and follow the instructions provided by the manufacturer.
For more question on quaternary ammonium click on
https://brainly.com/question/27147558
#SPJ11
How many grams of lithium are in 3.50 moles of lithium
Answers
what are 3 forces that can cause a physical change
Answers
Explanation:
Physical changes are caused by forces like motion, temperature, and pressure.
8.) 4.75 mol NaCl = a.) 0.0813 g (aqua/light green) b.) 278 g (dark blue) c.) 446 g (purple) g NaCl
Answers
Answer: C.) 446 g NaCl.
Explanation:
c.) 446 g NaCl. This is because 4.75 moles of NaCl is equal to 446 grams. This is calculated by multiplying the molar mass of NaCl (58.44 g/mol) by 4.75 moles. Hence, the answer is 446 g NaCl.
Observable matter makes up about what percentage of the universe?
05%
O 10%
O 50%
0 95%
Tutori
Previous Activity
avtorot void
K!
Answers
The universe as we know it is home to several galaxies, planets and stars. Hydrogen and helium were the most abundant elements present in the early universe. Heavier elements like carbon, oxygen, phosphorus and even metals were later formed through various fusion reactions in stars.
Nevertheless, matter makes up only 5% of the universe. Nearly 95% is referred to as 'dark matter' which is a subject of research in many scientific communities.
Answer: A) 5%
what is the purpose of adding edta to prepared foods? what is the purpose of adding edta to prepared foods? to complex trace metal ions that catalyze decomposition reactions to complex iron (iii) ions so they can catalyze protein decomposition on cooking to prevent dissolution of the container in the food when stored for long periods of time to aid in browning of the surface during cooking to keep ions such as ca2 in solution so the foods look good
Answers
The purpose of adding EDTA (ethylenediaminetetraacetic acid) to prepared foods is primarily to act as a preservative and maintain the food's appearance and quality. It achieves this by complexing trace metal ions, such as iron (III) and calcium ions (Ca2+), which can catalyze decomposition reactions, leading to spoilage and undesirable changes in the food.
EDTA forms stable complexes with these metal ions, preventing them from participating in reactions that can cause protein decomposition or alter the food's appearance. This helps to extend the shelf life of the food and maintain its visual appeal, which is crucial in the food industry.
Additionally, EDTA can help prevent the dissolution of the container in which the food is stored, further ensuring the safety and quality of the product.
It is important to note that EDTA is not used to aid in browning during cooking or to specifically keep ions such as Ca2+ in solution for aesthetic purposes. Its primary role is to preserve the food by preventing unwanted reactions and maintaining overall quality.
learn more about EDTA here: brainly.com/question/12990414
#SPJ11