Answers
Answer:
CCCCCCCCCCCCCCCCCCCC THATS THE ANSWER
Explanation:
Related Questions
1. Describe a situation in which you might need to convert the units of a measurement, and
what information you would need to do so.
Answers
Vinegar is sold at the grocery store with a concentration of 5.0 % acetic acid. How many grams of acetic acid are in 28 g of Vinegar?
Answers
White vinegar typically consists of 93%–96% water and 4–7% acetic acid. It can be used to cooking, bake, cleaning, and get rid of weeds. It can also help you lose weight and lower your blood sugar and cholesterol. Consumption is safe in moderation, but excessive consumption or when combined with certain medications could be harmful.
Apple cider vinegar is widely used in cooking and as a salad dressing because it contains acetic acid and nutrients like vitamins C and B vitamins. But at the same time, it's been utilized customarily as medication. It helps in losing weight.
Learn more about vinegar, here:
https://brainly.com/question/23700611
#SPJ1
Need answers asap!!!!!!!!!

Answers
The water cycle, also known as the hydrologic cycle, is the continuous movement of water on, above, and below the surface of the Earth.
What is the water cycle?The water cycle involves a series of physical processes, including evaporation, condensation, precipitation, and runoff, that work together to move water from one location to another and to maintain the balance of water on Earth.
The water cycle begins when water from oceans, lakes, rivers, and other bodies of water evaporates into the atmosphere due to the heat from the sun. As water vapor rises into the atmosphere, it cools and condenses into clouds. When the clouds become saturated with water vapor, precipitation occurs in the form of rain, snow, sleet, or hail.
Learn more about water cycle:https://brainly.com/question/31195929
#SPJ1
Do not look up the answer on google, because I know exactly what the answer is. If you answer right will give thanks and brainliest

Answers
Answer:
THE ANSWER IS CORRECT!!
Bacteria and archaea carry out the process of nitrogen fixation, which reduces atmospheric nitrogen, N2, to the biologically useful form NH3, also called ammonia. The nitrogenase complex consists of two proteins, reductase and nitrogenase, which catalyze the reactions of nitrogen fixation. Match each structural feature or function with the corresponding component of the nitrogenase complex.
a. also called the molybdenum—iron protein, or the MoFe protein
b. transfers electrons from a donor, such as frrredoxin, to the other component.
c. Homodimer
d. Heterotetramer
1. Reductase
2. Nitrogenase
Answers
Answer:
a. Nitrogenase
b. Reductase
c. Reductase
d. Nitrogenase
Explanation:
Reductase is a enzyme which promotes chemical reduction for a substance. It is also known as iron protein as iron is main component in reductase. Nitrogenase are molybdenum because they sulfur as co factor
How many moles of ammonia gas (NH3) would occupy a 0.43L container at STP
Answers
Answer:
0.0192 moles
Explanation:
One mole of any gas occupies 22.414 L at S.T.P
setting up mole and volume ratio;
mole : volume
1 : 22.414
X : 0.43
X = 0.43/22.414
X = 0.0192 moles
Which is the larger atom S or K
Answers
Answer:
I'm pretty sure k
Explanation:
I'm not for sure I'm trying to help
Answer:
Potassium
Explanation:
Atomic size decreases across a Period, from left to right as we face the Table, and INCREASES down a Group, a column of the Periodic Table.
10.Give the possible values for the magnetic quantum number for each of the following orbitals (a) 4p: (b) 3d: (c) 2s (d) 5P
Answers
Answer:
(a) 4p: ml = -3, -2, -1, 0, +1, +2, +3
(b) 3d: ml = -2, -1, 0, +1, +2
(c) 2s: ml = -1, 0, +1
(d) 5p: ml = -4, -3, -2, -1, 0, +1, +2, +3, +4
Explanation:
Manetic quantum numbers determine the spatial orientation and number of orbitals in the subshell. ml is from -l to +l.
3. A Wilkinson’s catalyst is widely used in the hydrogenation of alkenes. Show a catalytic cycle, including: i. chemical structure of the catalyst, with complete stereochemistry ii. molecular geometry of catalyst iii. type of reactions involved iv. the appropriate starting material, reagent and solvent v. major and minor end-products vi. all intermediates, for each reaction stated in (iii)
Answers
We can see here that the catalytic cycle for the hydrogenation of alkenes using Wilkinson's catalyst involves several steps.
What are the steps involved?Here's an overview of the catalytic cycle, including the necessary details:
i. Chemical structure of the catalyst:
Wilkinson's catalyst, also known as chloridotris(triphenylphosphine)rhodium(I), has the following chemical structure: [RhCl(PPh3)3]
ii. Molecular geometry of the catalyst:
The Wilkinson's catalyst has a trigonal bipyramidal geometry around the rhodium center. The three triphenylphosphine (PPh3) ligands occupy equatorial positions, while the chloride (Cl) ligand occupies an axial position.
iii. Type of reactions involved:
The catalytic cycle involves several reactions, including:
Oxidative addition: The rhodium center undergoes oxidative addition, reacting with molecular hydrogen (H2) to form a dihydride intermediate.Alkene coordination: The alkene substrate coordinates to the rhodium center, forming a π-complex.Hydrogenation: The coordinated alkene undergoes hydrogenation, resulting in the addition of hydrogen atoms to the double bond and formation of a metal-alkyl intermediate.Reoxidation: The metal-alkyl intermediate reacts with a hydrogen molecule to regenerate the rhodium dihydride species.iv. Starting material, reagent, and solvent:
The starting material is an alkene, and the reagent is Wilkinson's catalyst ([RhCl(PPh3)3]). The reaction is typically carried out in a suitable solvent, such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF).
v. Major and minor end-products:
The major end-product of the hydrogenation reaction is the fully saturated alkane, resulting from the addition of hydrogen across the double bond. The minor end-product may include cis- or trans-configured alkanes if the original alkene substrate possesses geometric isomers.
vi. Intermediates:
The intermediates in the catalytic cycle include:
Rhodium dihydride complex: [RhH2(PPh3)3]Alkene-Rhodium π-complex: [Rh(η2-alkene)(PPh3)3]Metal-alkyl intermediate: [Rh(alkyl)(PPh3)3]These intermediates play a crucial role in facilitating the hydrogenation reaction and enabling the catalytic cycle to proceed.
Learn more about Wilkinson’s catalyst on https://brainly.com/question/31972308
#SPJ1
What is the chemical name of the compound Na2CO3? Use the list of polyatomic ions and the periodic table to help you answer.
A.
sodium carbon oxide
B.
sodium carbonate
C.
sodium(II) carbonate
D.
sodium oxalate
Answers
Answer:
sodium carbonate
Explanation:
sodium carbonate
In the combustion of hydrogen gas, hydrogen reacts with oxygen from the air to form water vapor. hydrogen+oxygen⟶water
If you burn 46.2g of hydrogen and produce 413g of water, how much oxygen reacted?
mass of oxygen:
Answers
Answer:
ok, here is your answer
Explanation:
AI-generated answer
To find the mass of oxygen that reacted, we need to use the Law of Conservation of Mass, which states that in a chemical reaction, the mass of the reactants equals the mass of the products.
First, we need to find the number of moles of hydrogen that reacted:
Molar mass of hydrogen (H₂) = 2.016 g/mol
Number of moles of H₂ = mass/molar mass = 46.2 g/2.016 g/mol = 22.92 mol
Next, we need to use the balanced chemical equation to find the number of moles of water produced:
hydrogen + oxygen → water
2H₂ + O₂ → 2H₂O
From the equation, we can see that for every 2 moles of H₂, 1 mole of O₂ is required to produce 2 moles of H₂O. Therefore, the number of moles of O₂ required to produce 22.92 moles of H₂O is:
Number of moles of O₂ = 1/2 x 22.92 mol = 11.46 mol
Finally, we can find the mass of oxygen that reacted by using its molar mass:
Molar mass of oxygen (O₂) = 32.00 g/mol
Mass of oxygen = number of moles x molar mass = 11.46 mol x 32.00 g/mol = 366.72 g
Therefore, the mass of oxygen that reacted is 366.72 g.
mark me as brainliestwhat are all of the living and nonliving things in an area called?
Answers
Math the Definitions! Please & Thank You :) Screenshot Attached.
SOMEONE HELP!
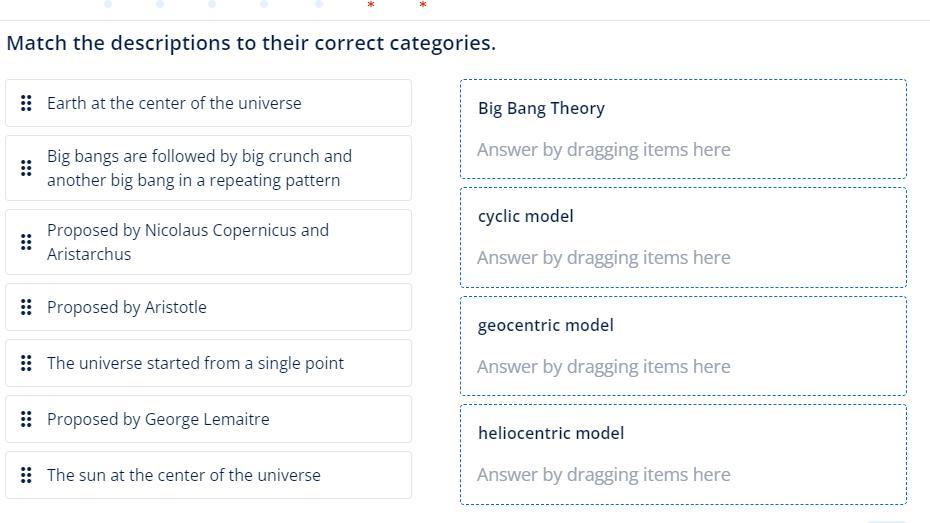
Answers
After analysing the given options and data we conclude that the matching of the given options as
1) Proposed by Nicolaus Copernicus and Aristarchus - heliocentric model
2) Proposed by Aristotle - 3) geocentric model
3) geocentric model - A) Earth at the center of the universe
4) The universe started from a single point - C) Big Bang Theory
5) Proposed by George Lemaitre - C) Big Bang Theory
6) The sun at the center of the universe - 3) geocentric model
7) heliocentric model - 6) The sun at the center of the universe
The Big Bang Theory is a scientific explanation regarding the original creation of the universe. The theory is based on several key assumptions, one of which is the isotropy of the universe. This assumption projects that the universe, more or less, appears the same in all directions of time and space.
Another key theory is that of cosmic inflation, which projects why the universe is so symmetrical on such a significant scale.
To learn more about Big bang theory
https://brainly.com/question/6841128
#SPJ1
The picture shows a scientist collecting data from an ice core whats the answer giving brainliest PLEASE HELP

Answers
Answer:
I’m going with her it c
Explanation:
Answer:
it maybe C
Explanation:
If an equilibrium mixture of the three gases at 600K contains 2.92*10^-2 M COCH(g) and 1.76*10^2 M CO, what is the equilibrium
concentration of Cl2?

Answers
Answer:
C
Explanation:
In which case are the white balls the maximum distance, and the maximum angle apart?
Answers
The maximum distance between the two white balls is 2R, and the maximum angle between them is 180 degrees (or π radians).
How to solveMaximum distance between the balls:
The maximum distance between the two white balls will be achieved when they are placed at opposite ends of a diameter of the circular region.
In this case, the distance between them will be equal to the diameter of the circle, which is 2R.
Maximum angle between the balls:
To find the maximum angle between the two balls, imagine the center of the circle as the vertex of the angle, and the positions of the two balls as the endpoints of the angle's two sides.
Since the balls are located at opposite ends of a diameter, the angle formed will be a straight angle, which is 180 degrees (or π radians).
So, the maximum distance between the two white balls is 2R, and the maximum angle between them is 180 degrees (or π radians).
Read more about max distance here:
https://brainly.com/question/2264671
#SPJ1
Two white balls, A and B, are placed on a flat surface inside a circular region of radius R. What is the maximum distance and maximum angle between the balls that can be achieved
Which of the following molecules would you expect to have the highest boiling point?
1
O Molecule 3
O Molecule 1
O Molecule 4
O Molecule 2
2
3
OH
O
4

Answers
The highest boiling point based on the data is option 4
What is the highest boiling point?Compared to alcohols of comparable molecular weight, carboxylic acids often have higher boiling temperatures. Between the hydrogen atoms of adjacent molecules and the oxygen in the carboxyl group of carboxylic acids, strong intermolecular hydrogen bonds can develop. Because it takes more energy to break the intermolecular interactions and change the substance from a liquid to a gas during boiling, these hydrogen bonds help materials have higher boiling temperatures.
Although carboxylic acids and alcohols are both capable of forming hydrogen bonds, carboxylic acids have higher boiling temperatures due to the extra carboxyl group that they contain.
Learn more about boiling point:https://brainly.com/question/1514229
#SPJ1
A 45.0 g sample of a metal at 85.6 °C is placed in 150.0 g of water at 24.6 °C. The final temperature of the system is 28.3 °C. Calculate the specific heat of the metal.
Answers
Answer:
904.014 j/kgk
Explanation:
Mass of metal = 45g
Temperature of metal = 85.6°
Mass of water = 150
Temperature of water = 24.6
Final temperature of system = 28.3
Heat lost by metal = Heat gained by water
m1 * c1 * dt = m2 * c2 * dt
Q = quantity of heat
Q = m*c*dt
dt = change in temperature
dt of water = 28.3 - 24.6 = 3.7
dt of metal = 85.6 - 28.3 = 57.3
Specific heat capacity of water, c = 4200
(45 * 10^-3) * c * 57.3 = (150 * 10^-3) * 4200 * 3.7
2.5785c1 = 2331
c1 = 2331 / 2.5785
= 904.01396
= 904.014 j/kgk
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
7.0 x 10 -3 mol of I2 in 100.00ml of solution
Answers
Given:
- Moles of I2: 7.0 x 10^(-3) mol
- Volume of solution: 100.00 mL (which is equal to 0.1000 L)
Molarity (M) = Moles of solute / Volume of solution in liters
Molarity = (7.0 x 10^(-3) mol) / (0.1000 L)
Molarity = 0.070 M
Therefore, the concentration of the I2 solution is 0.070 M.
If you take 35 mL of solution from (#2c) and add 0.225 L of water what is the concentration of the resulting solution in (% v/v)? (hint: you must determine the final volume first)
HELP
Answers
We want to find the concentration of the resulting solution of mixing 35mL of a given solution and 0.225 L of water.
We will find that the concentration is 13.5%.
First, we need to write both of these in the same units.
We know that:
1000 mL = 1L
Then:
35 mL = 0.035 L
So when we mix the solution and the water we end with a total volume of:
V = 0.035L + 0.225L = 0.260L
The concentration of solution will be equal to the quotient between the volume of solution we used (0.035 L) and the total volume of the mixture (0.260L)
We will get:
Concentration = (0.035L)/(0.260L) = 0.135
Now to write it as a percentage, we only need to multiply this by 100%.
0.135*100% = 13.5%
This means that the mixture has a concentration of the 13.5%.
If you want to learn more, you can read:
https://brainly.com/question/202460
A sample of a substance who only oxidizable material is tin in the +2 state, is titrated with a dichromate solution that was prepared by dissolving 1.226g of K₂Cr₂O₇ in enough water to give a volume of 250.00cm³. A 0.0821g sample of the substance required a volume of 23.90cm³ of the titrant to reach the equivalence point . The product of the oxidation of the tin in the +2 state is to tin in the +4 state . The reduction product of the dichromate , is chromium in the +3 state. Calculate the % of tin in the substance . ( NB: Assume the reaction to take place in acidic )
Answers
Given that, the sample of a substance who only oxidizable material is tin in the +2 state and we are meant to the determine the percentage composition of tin in the substance;
Then, the percentage composition of the tin in the substance is calculated as 172.84%
From the given information:
The molecular weight is the addition of all the atomic masses of a compound.
The molecular weight of K₂Cr₂O₇ is calculated as follows:
= (2 × 39.1) + (2 × 52) + (7 × 16)
= 294.2
The molecular weight of Sn²⁺ = 118.7
The oxidation half-reaction for Tin(ii) ion is as follows:
\(\mathbf{Sn^{2+}\to Sn^{4+}+2e^{-}}\)
From the above equation, Tin (ii) ion oxidizes to Tin (iV) ion
The reduction of K₂Cr₂O₇ In the acidic medium, reduce Cr₂ to Chromium Cr³⁺.
\(\mathbf{K_2 Cr_2O_7 + 14H^{+}+6e^- \to 2K^+ + 2r^{3+} + 7H_2O}\)
To calculate the percentage % of Tin in the sample, we must first know the Equivalent weight of each substance.
Calculating the equivalent weight (Eq. weight) of each substance in the samples, we have the following:
For K₂Cr₂O₇:
\(\mathbf{Eq. weight = \dfrac{molecular \ weight }{numbers \ of \ electrons \ lost \ or \ gained }}\)
\(\mathbf{Eq. weight \ of \ K_2Cr_2O_7 = \dfrac{294.2 }{6}}\)
\(\mathbf{Eq. weight \ of \ K_2Cr_2O_7 = 49.03 \ g/eq}\)
\(\mathbf{Eq. weight \ of \ Sn^{2+} = \dfrac{118.7 }{2}}\)
\(\mathbf{Eq. weight \ of \ Sn^{2+} =59.35 g/eq}\)
The next step is to determine the Normality of the Solution.
Normality refers to the mole number per liter of the solution
\(\mathbf{Normality \ of \ K_2Cr_2O_7 = \dfrac{1.226 \ g }{49.03}\times \dfrac{1000}{250}}\)
\(\mathbf{= \dfrac{4.904}{49.03} N}\)
So, 23.90 mL of \(\mathbf{ \dfrac{4.904}{49.03} N}\) of K₂Cr₂O₇ is required to be present in the solution.
= \(\mathbf{=23.90 mL \times \dfrac{4.904}{49.03 } \ of \ Sn \ ion}\)
Thus, the amount of Sn in the sample is calculated as:
\(= \mathbf{\dfrac{4.904}{49.03}\times \dfrac{59.35}{1000}\times 23.90 \ mL}\)
= 0.1419 g
Finally, the percentage composition of tIn (Sn) in the sample is:
\(\mathbf{= \dfrac{0.1419}{0.0821}\times 100\%}\)
= 172.84%
NOTE: A 0.0821g sample of the substance was given. Ideally, it should be 0.821g. But the calculation was based on the data given.
Therefore, from the above explanation, we can conclude that the percentage % of Sn in the sample is 172.84%
Learn more about Redox reactions here:
https://brainly.com/question/13978139?referrer=searchResults
Based on the equation below, how many grams of nitrogen gas will be produced from the decomposition of one mole of sodium aside? Use a molar mass of 28.0 grams for nitrogen gas.
Answers
Mass of Nitrogen= 42 grams
Further explanationGiven
one mole of Sodium azide
Required
mass of Nitrogen
Solution
Reaction
The decomposition of one mol of sodium azide :
2 N a N 3 ( s ) → 2Na ( s ) + 3 N2 ( g )
From the equation, the mol ratio of N a N 3 ( s ) : N2 ( g ) = 2 : 3, so mol N2 :
= 3/2 x mol N a N 3
= 3/2 x 1
= 1.5 moles
Mass of Nitrogen
= mol x molar mass
= 1.5 x 28 g/mol
= 42 grams
Help me please tell me and I try to make u brainliest!

Answers
The rate law expresses the relationship of the rate of a reaction to the rate constant and the concentration of the reactants raised to some powers for the general reaction.
aA+bB→cC+dD
Rate law takes the form
r=k[A]x[B]y
where x and y are number that must be determined experimentely k is the rate constant and [A] and [B] are concentration of A & B respectively.
Gaseous reaction A→B+C
follows first order kinetics concentration of A changes from A changes from 1M to 0.25M in 138.6 min.Find the rate of reaction when conc. of A is 0.1 M.
Answers
The rate law is the relationship between the rate of a reaction and the rate constant, as well as the concentration of reactants raised to various powers.
What is rate law ?In chemical kinetics, the rate law (also known as the rate equation) is a mathematical equation that describes the rate of a chemical reaction as a function of the concentrations of the reactants. The rate law provides a quantitative way of expressing how the rate of a reaction depends on the concentration of each reactant, as well as any other factors that may affect the reaction rate, such as temperature or the presence of a catalyst. The general form of a rate law is:
rate = k[A]^m[B]^n
where "rate" is the rate of the reaction, [A] and [B] are the concentrations of the reactants A and B, respectively, and k is the rate constant. The exponents m and n are the reaction orders with respect to A and B, respectively, and they determine how the rate of the reaction changes with changes in the concentration of each reactant.
0.072 M/min is the correct answer.
r2 = 0.2/5
r1/r2= [A1/A2]m
K=1/25
r= K×1/8= 0.072 M/min
The between relationship the rate of a reaction and the rate constant, as well as the concentration of the reactants raised to various powers for the overall process, is expressed by the rate law.
To learn more about the rate law, click the given link ;
brainly.com/question/7694417
#SPJ4
Trend of atomic number and atomic size of the elements when we move from left to right in different periods of periodic table
Answers
Answer:
The atomic size decreases with an increase in atomic number when we move from left to right.
Explanation: Hope it helps you:))))))
Have a great day.
write an equation for the proton transfer reaction that occurs when the following base reacts with water. draw curved arrows that show a mechanism for the proton transfer, and modify the given structures to draw the resulting products.
Answers
Refer the product in the image.The transfer of a proton (H+) in chemistry typically occurs through a mechanism known as an acid-base reaction.
This process involves the transfer of a proton from an acidic species (the proton donor) to a basic species (the proton acceptor), leading to the formation of a new acidic species and a new acid-base reactions species. In the final structure, the tetrahedral shape of the molecule and the absence of charge indicate that the proton transfer has resulted in the formation of a neutral species. In other words, the proton has been transferred from an acidic species to a basic species, neutralizing both species and resulting in a neutral compound with a tetrahedral shape. However, the general idea of the transfer of a proton from an acidic species to a basic species remains a key principle in understanding acid-base reactions.
Learn more about acid-base reactions here:
https://brainly.com/question/10467673
#SPJ4
Refer the below image to answer the Question:


The half reaction with a more positive standard reduction potential will
Answers
The half reaction with a more positive standard reduction potential will proceed spontaneously in a redox reaction
The half reaction with a more positive standard reduction potential will undergo reduction when compared to the half reaction with a more negative standard reduction potential.
Oxidation-reduction reactions, often known as redox reactions, are a set of chemical reactions that involve electron transfer between reactants. In a redox reaction, one reactant is oxidized, losing electrons, while the other reactant is reduced, gaining electrons.
The oxidation half-reaction is the process of losing electrons and increasing the oxidation number, whereas the reduction half-reaction is the process of gaining electrons and decreasing the oxidation number. The total reaction is referred to as the redox reaction.
Half-reaction:Half-reaction refers to the two parts of an oxidation-reduction reaction that happen separately. A half-reaction must always be either an oxidation reaction or a reduction reaction. It also describes the movement of electrons and hydrogen ions in an equation.
Know more about redox reaction here:
https://brainly.com/question/21851295
#SPJ8
Calculate the force needed to accelerate a 300 kg mass at 5
mls acceleration.
Answers
Explanation:
Force=mass *acceleration
F=300*5
f
F=1500N
The equilibrium constant for the reaction H2(g) + I2(g) 2HI(g) is 62.5 at 800 K. What is the equilibrium concentration of I2 if at equilibrium [HI] = 0.15 M and [H2] = 0.10 M? A. 0.15 MB. 4.8 × 10–2 MC. 3.6 × 10–3 MD. 0.20 ME. 2.4 × 10–2 M
Answers
The equilibrium concentration of I2 is 3.6 × 1\(0^{-4}\) M.
Equilibrium is a state of balance in a reaction. This means that the left hand side of the reaction is equal to the right hand side of the reaction. In short it means that the reactants are equal to the products formed. The input is equal to the output hence a state of equilibrium.
The equilibrium constant for the reaction H2(g) + I2(g) ⇌ 2HI(g) is given by:
Kc = ([HI]²)/([H2][I2])
We are given that Kc = 62.5 at 800 K, [HI] = 0.15 M, and [H2] = 0.10 M. We need to find the equilibrium concentration of I2.
Plugging in the given values into the equation for Kc, we get:
62.5 = (0.15²)/(0.10[I2])
Solving for [I2], we get:
[I2] = (0.15²)/(62.5 × 0.10)
[I2] = 0.00225/6.25
[I2] = 3.6 × 1\(0^{-4}\) M
More equilibrium questions can be obtained here:https://brainly.com/question/15181617
#SPJ11