Answers
Acid rain can be caused by all of the following except earthquakes.
Rain has a naturally acidic pH range of 5 to 6. This acidity is caused by sulfur dioxide, nitrogen oxide, and carbon dioxide, which react with oxygen gas and water in the atmosphere to form sulfuric, nitric, and carbonic acids. Acid rain is generated when the atmosphere is overly polluted by these oxides, notably sulfur and nitrogen oxides. Acid rain is the major cause of building deterioration, metal corrosion, and the breakdown of ecological balance.
Acid rain is defined as rain or any other form of precipitation that is unusually acidic, containing high quantities of hydrogen ions. Most water, including drinking water, has a neutral pH of 6.5 to 8.5, however acid rain has a pH lower than this, ranging from 4-5 on average.
To learn more about Acid rain visit:https://brainly.com/question/11543614
#SPJ9
Related Questions
2 NaClO3 → 2 NaCl + 3 O2
Calculate the mass of O2 produced as the result of the decomposition of 843 g of NaClO3.
Answers
Taking into account the reaction stoichiometry, 380.12 grams of O₂ are produced as the result of the decomposition of 843 g of NaClO₃.
Reaction stoichiometryIn first place, the balanced reaction iS:
2 NaClO₃ → 2 NaCl + 3 O₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
NaClO₃: 2 molesNaCl: 2 molesO₂: 3 molesThe molar mass of the compounds is:
NaClO₃: 106.45 g/moleNaCl: 58.45 g/moleO₂: 32 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
NaClO₃: 2 moles ×106.45 g/mole= 212.9 gramsNaCl: 2 moles ×58.45 g/mole= 116.9 gramsO₂: 3 moles ×32 g/mole= 96 gramsMass of O₂ formedThe following rule of three can be applied: if by reaction stoichiometry 212.9 grams of NaClO₃ form 96 grams of O₂, 843 grams of NaClO₃ form how much mass of O₂?
mass of O₂= (843 grams of NaClO₃× 96 grams of O₂) ÷212.9 grams of NaClO₃
mass of O₂= 380.12 grams
Finally, 380.12 grams of O₂ are formed.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
A 1.85-mole sample of H₂O2 weighs
(A) 33.3 amu
(B) 35.9 g
C) 62.9 g
(D) 1.85 g
E 33.3 g
Answers
Considering the definition of molar mass, the correct answer is option c): the mass of 1.85 moles H₂O₂ is 62.9 grams.
Definition of molar massThe molar mass of substance is a property defined as the amount of mass that a substance contains in one mole.
The molar mass of a compound is the sum of the molar mass of the elements that form it (whose value is found in the periodic table) multiplied by the number of times they appear in the compound.
Molar mass of H₂O₂In this case, you know the molar mass of the elements is:
O= 16 g/moleH= 1 g/moleSo, the molar mass of the compound H₂O₂ is calculated as:
H₂O₂= 2× 1 g/mole + 2× 16 g/mole
Solving:
H₂O₂= 34 g/mole
Mass of 1.85 moles H₂O₂You can apply the following rule of three: If by definition of molar mass 1 mole of the compound contains 34 grams, 1.85 moles of the compound contains how much mass?
mass= (1.85 moles× 34 grams)÷ 1 mole
mass= 62.9 grams
Finally, the mass of 1.85 moles H₂O₂ is 62.9 grams.
Learn more about molar mass:
brainly.com/question/5216907
#SPJ1
Which shows a triglyceride?

Answers
Answer:
A
Explanation:
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from tri- and glyceride). Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat.
The structure of option A shows a triglyceride.
What is triglyceride?Triglycerides are a kind of fat present in the blood. Triglycerides are the types of stored fats, which are not in use currently.
The structure of triglyceride is TRI esters containing glycerol bound to three fatty acid molecules.
Thus, the correct option is A.
Learn more about triglyceride
https://brainly.com/question/13840067
#SPJ2

how many grams of hexane(C6H14) would be need to make 562 of carbon dioxide
Answers
The mass of hexane, C₆H₁₄ that would be needed to produce 562 grams of carbon dioxide, CO2 is 183 g
How do I determine the mass of hexane, C₆H₁₄ needed?We'll begin by writing the balanced equation for the reaction.
2C₆H₁₄ + 19O₂ → 12CO₂ + 14H₂O
The following are obtained from the equation:Molar mass of C₆H₁₄ = 86 g/molMass of C₆H₁₄ from the balanced equation = 2 × 86 = 172 g Molar mass of CO₂ = 44 g/molMass of CO₂ from the balanced equation = 12 × 44 = 528 gFrom the balanced equation above,
528 g CO₂ were produced from 172 g of C₆H₁₄
With the above information, we shall determine the mass of hexane, C₆H₁₄ needed to produce 562 g CO₂. Details below:
From the balanced equation above,
528 g CO₂ were produced from 172 g of C₆H₁₄
Therefore,
562 g of CO₂ will react to produce = (562 × 172) / 528 = 183 g of C₆H₁₄
Thus, the mass of hexane, C₆H₁₄ needed is 183 g
Learn more about mass:
https://brainly.com/question/25824598
#SPJ1
The diagram shows four different locations in an atom.
2
Nucleus
3
7
4
Which locations are likely to have subatomic particles with the LEAST mass? (3 points)
a
1 and 2
b
2 and 3
с
1 and 4
Od
3 and 4
Answers
The locations with subatomic particles with the least mass are locations 1 and 4.
An atom is usually divided into two main regions:
The nucleus: This is shown in the diagram with the numbers 2 and 3, and contains protons and neutrons.The outer region: This region includes everything outside the nucleus, and in this region, it is expected to find electrons.The three sub-particles previously mentioned (neutrons, protons, and electrons) differ not only in their location but also in their mass. Indeed, electrons have a lower mass if compared to neutrons and protons.
Based on this, the outer region (locations 1 and 4) has sub particles with the least mass.
Learn more about atom in: https://brainly.com/question/13981855

Answer:
1 and 4
Explanation:
took the test
Just saying thank you person above you are very great and smart. You deserve a hugeeeeeeee thank you. So, thank you!!!!!!!!!
Science is a way of discovering what's in the natural world and how those things
A)
work today
B)
how they worked in the past
C)
how they are likely to work in the future
D)
work today, how they worked in the past, and how they are likely to work
in the future
Answers
Answer:
D
Explanation:
science shows patterns and anomalies in how the world works
Ammonia ( NH3, MM 17.031 g/mol) and hypobromite ( OBr−) react according to the following chemical reaction. 2NH3+3OBr−⟶N2+3Br−+3H2O Suppose 0.325 g of ammonia reacts with 14.5 mL of a hypobromite solution. Calculate the concentration of the hypobromite solution.
Answers
Answer:
1.98 M
Explanation:
Step 1: Write the balanced reaction
2 NH₃ + 3 BrO⁻⟶ N₂ + 3 Br⁻ + 3 H₂O
Step 2: Calculate the moles corresponding to 0.325 g of ammonia
The molar mass of NH₃ is 17.03 g/mol.
0.325 g × (1 mol/17.03 g) = 0.0191 mol
Step 3: Calculate the reacting moles of hypobromite
The molar ratio of NH₃ to BrO⁻ is 2:3. The reacting moles of hypobromite are 3/2 × 0.0191 mol = 0.0287 mol
Step 3: Calculate the molar concentration of the hypobromite solution
M = 0.0287 mol / 0.0145 L = 1.98 M
The concentration of the hypobromite solution (BrO⁻) needed for the reaction is 1.97 M
We'll begin by calculating the number of mole in 0.325 g of NH₃. This can be obtained as follow:
Mass of NH₃ = 0.325 g
Molar mass of NH₃ = 17.031 g/mol
Mole of NH₃ =?Mole = mass / molar mass
Mole of NH₃ = 0.325 / 17.031
Mole of NH₃ = 0.01908 moleNext, we shall determine the number of mole of BrO⁻ needed to react with 0.01908 mole of NH₃. This can be obtained as follow:
2NH₃ + 3BrO⁻ —> N₂ + 3Br⁻ + 3H₂O
From the balanced equation above,
2 moles of NH₃ reacted with 3 moles of BrO⁻.
Therefore,
0.01908 mole of NH₃ will react with = \(\frac{0.01908 * 3 }{2}\\\\\) = 0.02862 mole of BrO⁻.
Finally, we shall determine the concentration of the BrO⁻ solution.
Mole of BrO⁻ = 0.02862 mole
Volume of BrO⁻ solution = 14.5 mL = 14.5 / 1000 = 0.0145 L
Concentration of BrO⁻ =?Concentration = mole / Volume
Concentration of BrO⁻ = 0.02862 / 0.0145
Concentration of BrO⁻ = 1.97 MTherefore, the concentration of the hypobromite solution (BrO⁻) is 1.97 M
Learn more: https://brainly.com/question/2161535
Polyethylene is 86.0% C and 14.0%
H. Determine the empirical formula of the compound.
Percent to Mass: How many grams of C/and Hare present in 100.0 g?
Answers
The empirical formula of polyethylene can be determined by converting the given percentages of carbon (C) and hydrogen (H) into grams. To find the grams of each element, we assume a 100.0 g sample of polyethylene.
For carbon:
Mass of carbon = 86.0% × 100.0 g = 86.0 g
For hydrogen:
Mass of hydrogen = 14.0% × 100.0 g = 14.0 g
Therefore, in a 100.0 g sample of polyethylene, there are 86.0 grams of carbon and 14.0 grams of hydrogen.
The empirical formula of a compound represents the simplest whole-number ratio of atoms present in the compound. To determine the empirical formula, we need to find the ratio of carbon to hydrogen in terms of moles.
First, we convert the masses of carbon and hydrogen into moles using their respective molar masses. The molar mass of carbon is approximately 12.01 g/mol, and the molar mass of hydrogen is approximately 1.008 g/mol.
Number of moles of carbon = 86.0 g / 12.01 g/mol ≈ 7.162 mol
Number of moles of hydrogen = 14.0 g / 1.008 g/mol ≈ 13.89 mol
Next, we divide the number of moles of each element by the smallest number of moles to get a simplified ratio.
Carbon: Hydrogen ≈ 7.162 mol : 13.89 mol ≈ 1 : 1.939
Since we want to express the ratio in whole numbers, we multiply both sides by 2 to get a whole number ratio.
Carbon: Hydrogen ≈ 2 : 3.878
Rounding to the nearest whole number, we find that the empirical formula of polyethylene is CH₂.
for such more questions on hydrogen
https://brainly.com/question/24433860
#SPJ8
g calculate the quantity of energy produced per gram of uranium 235 for the neutron induced Fusion of uranium
Answers
Answer:
Energy released by 1 g of U-235 = 7.603 * 10¹⁰ J
Explanation:
Uranium-235, U-235 undergoes neutron-induced fission to give the following products:
1 neutron + ²³⁵U --> ¹⁴¹Ba + ⁹²Kr + 3 neutrons
Masses of reactants and products:
neutron = 1.009 amu, uranium-235 = 235.044 amu, Ba-141 = 140.910 amu, Kr-92 = 91.910 amu
mass defect = mass of reactants - mass of products
mass defect = (235.044 + 1.009) - (140.910 + 91.910 + 3 * 1.009)
mass defect = 236.053 - 235.847 = 0.206 amu
1 amu = 1.6 * 10⁻²⁷ kg
Using E = mc²
E = 0.206 * 1.6* 10⁻²⁷ kg * (3 * 10⁸ m/s)² = 2.966 * 10⁻¹¹ J
therefore, 1 atom of U-235 releases 2.966 * 10⁻¹¹ J of energy
energy released by 1 g of U-235 can be calculated as follows:
1 mole or 253 g of U-235 contains 6.02 * 10³ atoms
1 g of U-235 will contain 6.02 * 10³/235 = 2.563 * 10²¹ atoms
Energy released by 1 g of U-235 = 2.563 * 10²¹ * 2.966 * 10⁻¹¹ J = 7.603 * 10¹⁰ J
1.0 mole of a gas is enclosed in a 12.3 liter cylinder with a moveable piston at 300 K and 2.0 atm. Half of the gas is removed, leaving 0.50 mole in the cylinder and the system is warmed to 900 K. The cylinder changes volume to maintain constant pressure. What is the volume in the final system?1.0 mole of a gas is enclosed in a 12.3 liter cylinder with a moveable piston at 300 K and 2.0 atm. Half of the gas is removed, leaving 0.50 mole in the cylinder and the system is warmed to 900 K. The cylinder changes volume to maintain constant pressure. What is the volume in the final system?
Answers
The final volume of the system, given that half of the gas is removed, leaving 0.50 mole in the cylinder and the system is warmed to 900 K is 18.45 liters
How do I determine the final volume of the system?From ideal gas equation, we have
PV = nRT
Rearrange
V / nT = R/ P
R / P = Constant
Thus, we have
V₁ / n₁T₁ = V₂ / n₂T₂
Where
V₁ and V₂ are initial and final volumen₁ and ₂ are initial and final moleT₁ and T₂ are initial and final temperatureWith the above formula, we can obtain the final volume of the system as follow:
Initial mole (n₁) = 1 moleInitial volume of gas (V₁) = 12.3 litersInitial temperature (T₁) = 300 KPressure = ConstantFinal mole (n₂) = 0.5 moleFinal temperature (T₂) = 900 KFinal volume (V₂) = ?V₁ / n₁T₁ = V₂ / n₂T₂
12.3 / (1 × 300) = V₂ / (0.5 × 900)
Cross multiply
1 × 300 × V₂ = 12.3 × 0.5 × 900
300 × V₂ = 5535
Divide both side by 300
V₂ = 5535 / 300
V₂ = 18.45 liters
Thus, the final volume of the system is 18.45 liters
Learn more about volume:
https://brainly.com/question/14560487
#SPJ1
Which of the following indicates how fast something is moving?
1. gravity
2. force
3. speed
4. inertia
Answers
Answer:
Speed.
Explanation:
Gravity pulls objects towards earth's core. Force is the strength applied to something being pulled or pushed. Inertia is for something to stay still. Ruling out all of those, speed is the answer.
Answer:
Speed
Explanation:
Speed tells us how fast something or someone is travelling. You can find the average speed of an object if you know the distance travelled and the time it took. The formula for speed is speed = distance ÷ time.
3,3-dibromo-4-methylhex-1-yne
Answers
Explanation:
see the attachment. hope it will help you...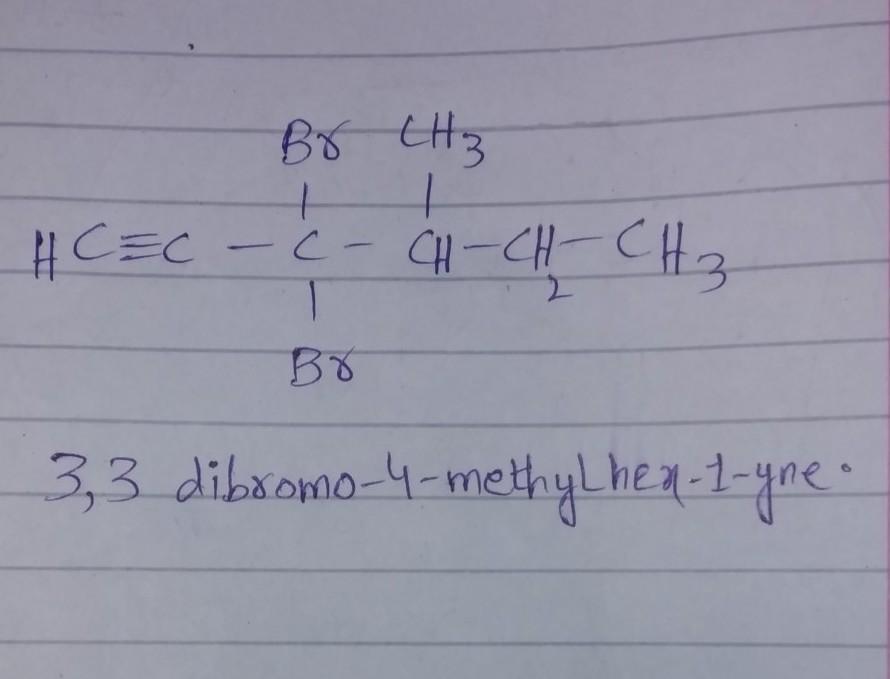
In a science demonstration, a teacher mixed zinc (Zn) with hydrogen chloride (HCl) in a flask and quickly attached a balloon over the mouth of the flask. Bubbles formed in the solution and the balloon inflated.
What most likely occurred during this demonstration?
a.The Zn and HCl both retained their identity.
b.Either Zn or HCl, but not both, retained its identity.
c.Evaporation of one of the substances occurred.
d.One or more new substances formed.
Answers
Answer:
a. The Zn and HCl both retained their identity.
Spirit of Australia, a hydroplane boat, made speed records by traveling 239 miles in 0.75 hours (45 minutes). What is its record- breaking speed?
Answers
239 miles per 0.75 hours
239 miles / 45 minutes = 5.3111 miles per minute
5.3111 miles per minutes X 60 minutes per hour = 318.67 miles per hour ~ 319 mph
Answer:
the answer is 319 mph
Explanation:
you do 239/.75 (45 minutes will get you a different answer)
. How many grams of lithium are formed from the reaction if you begin with 89.5
grams of copper?
Cu
+
Li2S --> 2 Li +
CUS
Answers
Answer:
179g
Explanation:
Molar ratio for Copper : Lithium is
1:2
If there are 89.5g of copper
Grams for Lithium= 89.5 × 2 = 179g of Lithium
The amount of lithium are formed from the reaction if you begin with 89.5 grams of copper is 19.6 grams.
How do we convert mass into moles?Mass can be converted into moles by using the below equation as:
n = W/M, where
W = given mass
M = molar mass
Moles of 89.5g of copper = 89.5g / 63.5g/mol = 1.4 mol
Given chemical reaction is:
Cu + Li₂S → 2Li + CuS
From the stoichiometry of the reaction, it is clear that
1.4 mole of Cu = produces 1.4 mole of CuS
1.4 mole of CuS = produced by 1.4 mole of Li₂S
1.4 mole of Li₂S = produces 2×1.4=2.8 moles of Li
Mass of 2.8 moles of Li = (2.8mol)(7g/mol) = 19.6g
Hence resultant mass of lithium is 19.6g.
To know more about mass & moles, visit the below link:
https://brainly.com/question/24631381
#SPJ2
The satellite image above shows the San Francisco area along the West Coast. What feature is marked by "X"?
A. A bay
B. A fresh water lake
C. A mountain
D. A volcano

Answers
A bag because it broad inlet of the sea where the land curves inwards
What is the best method of separating the mixture of sand and fine salt?
Answers
By using filtration, the sand and fine salt can be effectively separated based on their difference in particle size, providing a clean separation of the two components.
Filtration is a separation technique that takes advantage of the difference in particle size between sand and salt. It involves passing the mixture through a porous material, such as filter paper or a filter funnel, which allows the liquid (saltwater) and small salt particles to pass through while retaining the larger sand particles.
Here's how the filtration process can be carried out:
1. Set up a filter apparatus with a funnel and filter paper or a filter flask.
2. Place the mixture of sand and salt in a beaker or a flask.
3. Slowly pour the mixture into the filter paper or funnel, allowing the liquid (saltwater) to pass through while retaining the sand on the filter paper.
4. Once the liquid has passed through completely, the sand will be left behind on the filter paper or in the filter flask.
5. Carefully remove the sand from the filter paper or filter flask, and the saltwater solution can be collected separately.
For more such questions on filtration
https://brainly.com/question/29756050
#SPJ8
Given the reaction: Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
The reaction occurs more rapidly when a 10-gram sample of Mg is powdered rather than in one piece, because powdered
Mg has
1. less surface area
2. more surface area
3. a lower potential energy
4. a higher potential energy
Answers
how many valence electrons in an oxygen atom?
Answers
Answer:
6 valence electrons
Explanation:
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell.
what is the function or protons, neutrons, and electrons?
someone help please
Answers
Answer:
An atom is composed of them
Explanation:
Answer:
Each element has a different amount of protons. Neutrons are responsible for combining with protons in the nucleus together to be held by the strong force. Too many neutrons in an atom will result in radioactive decay due to the neutrons' force overpowering the strong force. Electrons are the weirdest particles.
Explanation:
sorry if thats too much to read but i hope that helps :)
how many molecules are in 0.610 moles of neon gas?
Answers
A 600. mL beaker has an inner diameter of 77.0 mm. What is the vertical distance between the 100. mL marks on the side of the beaker
Answers
Answer:
\(h=12.9cm\)
Explanation:
Hello!
In this case, since we can consider the beaker until the 100-mL mark as a cylinder, we can use the following equation to relate its diameter, vertical distance or height and volume:
\(V=\pi h\frac{d^2}{4}\)
Thus, since we know the diameter, volume (which is equivalent to 600 cm³) and π, we can plug in to obtain:
\(600cm^3=\pi *h*\frac{(77.0mm)^2}{4}\)
It means it is necessary to take the mm to cm and solve for h:
\(h=\frac{600cm^3}{\pi*\frac{(7.70cm)^2}{4}} \\\\h=12.9cm\)
Best regards!
The distance between each 100 mL mark is 2.15 cm.
The volume of a cylinder is obtained using the formula;
V = πr^2h
Now, we have the following information;
Volume of the cylinder = 600. mL or 600 cm^3
Diameter of the cylinder = 77 mm or 7.7 cm
Radius of the cylinder = 7.7/2 = 3.85 cm
Height of the cylinder = h
Hence;
600 = 3.142 × ( 3.85 )^2 × h
h = 600/3.142 × ( 3.85 )^2
h = 12.88 cm
There are six 100 mL marks on the beaker, the distance between each 100 mL mark = 12.88 cm/6 = 2.15 cm
Learn more about volume: https://brainly.com/question/12748872
what mass of glucose c6h12o6 would be required to prepare 5000 mL of a 0.215 M solution
Answers
Approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M.
To determine the mass of glucose (C6H12O6) required to prepare a 0.215 M solution in 5000 mL, we need to use the formula:
Molarity (M) = moles of solute / volume of solution (in liters)
First, let's convert the volume of the solution from milliliters (mL) to liters (L):
5000 mL = 5000/1000 = 5 L
Now, we can rearrange the formula to solve for moles of solute:
moles of solute = Molarity (M) x volume of solution (L)
moles of solute = 0.215 M x 5 Lmoles of solute = 1.075 mol
Since glucose (C6H12O6) has a molar mass of approximately 180.16 g/mol, we can calculate the mass of glucose using the equation:
mass of solute = moles of solute x molar mass of solute
mass of glucose = 1.075 mol x 180.16 g/mol
mass of glucose = 194.0 g (rounded to three significant figures)
Therefore, approximately 194.0 grams of glucose (C6H12O6) would be required to prepare a 5000 mL solution with a concentration of 0.215 M. It's important to note that the molar mass of glucose used in this calculation may vary slightly depending on the level of precision required.
For more such questions on glucose visit:
https://brainly.com/question/397060
#SPJ8
How does alternate freezing and thawing of water cause weathering to occur?
A Water expands when it freezes and cracks rocks open Water expands when it freezes and cracks rocks open
B Thawing causes rock particles to move from place to place Thawing causes rock particles to move from place to place
C Freezing chemically alters the rock surfaces
Answers
Freezing leads to weathering because water expands when it freezes and cracks rocks open Water expands when it freezes and cracks rocks open
What is weathering?The term weathering has to do with the process of the breakdown of the rocks to give rise to the soil. Now there are are various ways in which the process of weathering could be able to take place. One of the ways that the process of weathering can take place is what we call physical weathering.
In the process of physical weathering, we are looking at some kind of physical pressure which would lead to the breakdown of the rock. This is what makes it different from the chemical and the mechanical forms of weathering.
Thus, the fact that water would expand when we freeze it would mount pressure on the rock and then cause it to weather and form soil.
Learn more about weathering:https://brainly.com/question/14426457
#SPJ1
A tank of nitrogen has a volume of 14.0 L and a pressure of 1 atm. Find the volume of the nitrogen when its pressure is changed to 0.8 atm while the temperature is held constant .
Answers
Answer:
17.5 L
Explanation:
Step 1: Given data
Initial volume of the tank of nitrogen (V₁): 14.0 LInitial pressure of nitrogen (P₁): 1 atmFinal volume of the tank of nitrogen (V₂): ?Final pressure of nitrogen (P₂): 0.8 atmStep 2: Calculate the final volume of the nitrogen
We have a gas that undergoes a change at a constant temperature. If we assume an ideal behavior, we can calculate the final volume of the nitrogen using Boyle's law.
P₁ × V₁ = P₂ × V₂
V₂ = P₁ × V₁/P₂
V₂ = 1 atm × 14.0 L/0.8 atm = 17.5 L
A gas has a volume of 53.0 ml and is heated until it reaches a temperature of 510 K and a volume of 0.250 L, What was its original temperature?
Answers
The original temperature of the gas, given the data from the question is 108.12 K
Data obtained from the questionThe following data were obtained from the question:
Original volume (V₁) = 53 mLNew temperature (T₂) = 510 KNew volume (V₂) = 0.250 L = 0.25 × 1000 = 250 mLOriginal temperature (T₁) =?How to determine the original temperatureThe original temperature of the gas can be obtained as follow:
V₁ / T₁ = V₂ / T₂
53 / T₁ = 250 / 510
Cross multiply
T₁ × 250 = 53 × 510
T₁ × 250 = 27030
Divide both sides by 250
T₁ = 27030 / 250
T₁ = 108.12 K
Thus, the original temperature of the gas is 108.12 K
Learn more about gas laws:
https://brainly.com/question/6844441
#SPJ1
What is the controlled variable of your experiment?
Your answer
Answers
Answer:
The controlled variable of an experiment is the one thing that stays the same in an experiment.
Explanation:
An example would be : if I have two pennies, both dunked in water, but than I change one to be dunked in vinegar, the one dunked in water still is the constant or the controlled variable.
PLEASE HELP, WILL MARK BRAINLIEST. The two boron atoms listed in the table are isotopes of boron. The two carbon atoms and the two oxygen atoms are also called isotopes. Based on the patterns in the particle compositions of these atoms, write a definition for “isotope.”

Answers
Don’t really know ‘bout this one, but here’s something I found.
Answer:
Isotopes are various forms of an element that have the same number of protons but a different number of neutrons. Some elements, such as carbon, potassium, and uranium, have multiple naturally-occurring isotopes. Isotopes are defined first by their element and then by the sum of the protons and neutrons present.
Carbon-12 (or 12C) contains six protons, six neutrons, and six electrons; therefore, it has a mass number of 12 amu (six protons and six neutrons).
Carbon-14 (or 14C) contains six protons, eight neutrons, and six electrons; its atomic mass is 14 amu (six protons and eight neutrons).
While the mass of individual isotopes is different, their physical and chemical properties remain mostly unchanged.
What is the molarity of a solution that contains 4.53 moles of lithium nitrate (LiNO3) in 2.85 liters of solution
Answers
Answer:
M= ml - 45301 - 11.59M.
Explanation:
Why are diamonds unevenly distributed on Earth?
Soil forms only in places where rock particles are deposited.
Soil forms only in places where larger rocks are broken into smaller rocks.
Soil forms only within the Earth under heavy pressure.
Soil forms in the air and is then deposited mainly in places that get a lot of rain.
Answers
Answer:
Why aren't diamonds found evenly distributed on Earth? Diamonds are only formed under specific conditions that involve geological processes, such as volcanic activities and plate movements. Since these conditions and processes only take place in certain areas, you can only find diamonds in these special places.
Explanation: