3. What is SI system? Why has SI system been developed? Give reasons
Answers
P.S PLS MARK AS BRAINLIST

Related Questions
For PO43 -, phosphate ion, draw the Lewis structure (by counting valence electrons of each atom), determine the a) electron-domain geometry b) molecular geometry c) hybridization d) show the angle between the bonds in a drawing
Answers
The Lewis structure for PO43- (phosphate ion) would be:O = P = O|O-To determine the electron-domain geometry, we need to count the number of electron domains around the central phosphorus atom.
In this case, we have 4 single bonds and 1 lone pair of electrons, so there are a total of 5 electron domains. This corresponds to a trigonal bipyramidal electron-domain geometry.Next, we need to determine the molecular geometry, which takes into account the lone pairs of electrons. In this case, the lone pair of electrons will occupy one of the equatorial positions, giving us a molecular geometry of tetrahedral.The hybridization of the central phosphorus atom can be determined based on the electron-domain geometry. Since we have 5 electron domains, the hybridization would be sp3d.
Finally, we can draw the molecule with the bond angles included. The three equatorial bonds are all at 120 degrees to each other, while the axial bonds are at 90 degrees to the equatorial bonds. The angle between the axial bonds is also 120 degrees. Here's a rough sketch:
O
|
O-P-O
|
O O
Learn more about phosphate ion here:https://brainly.com/question/19197985
#SPJ11
a researcher performs the above reaction starting with 1.50 g of the alkene substrate, 3.75 g of pyridinium tribromide, and 20.0 ml of acetic acid. only one product is observed. 2. what is the limiting reagent? b. what is the theoretical yield? c. if the researcher obtains 3.05 g of pure product, what is the percent yield?
Answers
For a chemical reaction present in above figure,
a) The limiting reagent is Alkene.
b) The theoretical yield of product is 3.32 g.
c) The percentage yield is about 92%, when the actual yield is 3.05 g.
We have a reaction starting with 1.50 g of the alkene substrate, 3.75 g of pyridinium tribromide, and 20.0 ml of acetic acid and produce a single product is obtained. See the above figure 2.
Molecular mass of reactant = 10× 12 + 12×1 = 132 g
Molecular mass of product = 10× 12 + 2× 80 + 12 × 12 =292 g
a) The limiting reagent is alkene in this reaction.
According to reaction, 132g reactant -> 292 g product
so, 1g reactant --> 292/132 g product
1.50 g reactant ->1.50( 292/132)g product
= 3.32 g product
b) The theoretical yield of product is 3.32 g in this reaction.
c) Percent Yield is calculated as the actual yield divided by the theoretical yield then multiply the resultant with 100.
Now, 3.05 g of pure product, that is actual yield of product= 3.05 g
Percentage of yield= ( 3.05 /3.32 )× 100
= 91.86% ~ 92%
Hence, required value is 92%.
For more information about percent yield, visit :
https://brainly.com/question/14714924
#SPJ4
Complete question:
The above figure complete the question
a researcher performs the above reaction starting with 1.50 g of the alkene substrate, 3.75 g of pyridinium tribromide, and 20.0 ml of acetic acid. only one product is observed. 2. what is the limiting reagent? b. what is the theoretical yield? c. if the researcher obtains 3.05 g of pure product, what is the percent yield?


A 10.0-mL solution of 0.300 M NH3 is titrated with a 0.100 M HCl solution. Calculate the pH after the following additions of the HCl solution: (a) 0.0 mL, (b) 10.0 mL, (c) 30.0 mL, (d) 40.0 mL
Answers
The pH values of the solution after the addition of various amounts of acids are as follows:
(a) When 0.0 mL HCl is added; pH = 11.62
(b) After adding 10.0 mL of 0.100 M HCl; pH = 12.38
(c) After adding 30.0 mL of 0.100 M HCl; pH = 14
(d) After adding 40.0 mL of 0.100 M HCl; pH = 2.70
What are the pH values of the solution after the addition of various amounts of acids?The equation of the reaction between NH₃ and HCl is:
NH₃ + HCl → NH₄Cl
Before the addition of HCl:
The concentration of NH₃ solution = 0.300 M
The initial concentration of OH- can be calculated from the Kb of NH₃ :
Kb = [NH₄⁺][OH⁻] / [NH₃]
1.8 x 10⁻⁵ = x² / 0.300
x = 0.0024 M
The initial concentration of OH⁻ is 0.0024 M, and the initial pH of the solution ill be:
pH = 14 - pOH
pH = 14 - (-log[OH-])
pH = 11.62
(a) When 0.0 mL HCl is added;
pH = 11.62
(b) After adding 10.0 mL of 0.100 M HCl;
moles of HCl added = (0.100 mol/L) x (0.0100 L)
moles of HCl added = 0.00100 mol
The moles of NH₃ initially present = (0.300 mol/L) x (0.0100 L)
moles of NH₃ initially present = 0.00300 mol
the mole ratio of HCl and NH₃ = 1:1
The remaining moles of NH₃ = 0.00300 mol - 0.00100 mol
remaining moles of NH₃ = 0.00200 mol
The volume of the solution is now 10.0 mL + 10.0 mL = 20.0 mL or 0.0200 L.
The new concentration of NH₃ = 0.00200 mol / 0.0200 L
concentration of NH₃ = 0.100 M
The concentration of OH- can be calculated from the Kb of NH₃ using the new concentration of NH4+:
Kb = [NH4+][OH-] / [NH3]
1.8 x 10⁵ = x² / 0.100
x = 0.0042 M
The new concentration of OH- is 0.0042 M, and the new pH will be:
pH = 14 - (-log[OH-])
pH = 12.38
(c) After adding 30.0 mL of 0.100 M HCl;
the volume of the solution is 40.0 mL or 0.0400 L
The moles of HCl added = (0.100 mol/L) x (0.0300 L)
moles of HCl added = 0.00300 mol
moles NH3 reacted = 0.00300 mol - 0.00300 mol
moles NH3 reacted = 0.00000 mol
[NH₄⁺] remaining [OH⁻] = 0
The concentration of OH- is 0 M, so the pH = 14 - (-log[OH-])
pH = 14
(d) After adding 40.0 mL of 0.100 M HCl;
moles HCl = (0.100 mol/L) x (0.0400 L)
moles HCl = 0.00400 mol
The volume of solution = 0.050 L
All NH₃ has reacted and the remaining moles of HCl = (0.00400 mol - 0.00300 mol) / 0.0500 L
the remaining moles of HCl = 0.00200 M
The concentration of H₃O⁺ can be calculated from the concentration of HCl:
0.00200 M HCl produces 0.00200 M H₃O⁺
pH = -log[H₃O⁺]
pH = -log(0.00200)
pH = 2.70
Learn more about pH at: https://brainly.com/question/172153
#SPJ1
what happens when solid sodium chloride is mixed with water
Answers
When solid sodium chloride is mixed with water, it dissolves and forms a solution. Sodium chloride, also known as table salt, is an ionic compound that consists of positively charged sodium ions and negatively charged chloride ions.
When it is added to water, the water molecules surround each ion, separating them from each other and dissolving them. The ions become hydrated as they are surrounded by water molecules. The dissolution of sodium chloride in water is an exothermic process, meaning it releases heat. This is because when the ions are separated from each other, they release energy that was previously holding them together. The resulting solution is a clear, colorless liquid that conducts electricity due to the presence of ions.
Overall, the solid sodium chloride is transformed into a solution when mixed with water.
To know more about solution visit-
https://brainly.com/question/1616939
#SPJ11
which need or want does this technology meet

Answers
Answer:
B. It allows people to rapidly heat items.
Explanation:
because it's a microwave...
Answer:
B. It allows people to rapidly heat items.
I am a bit skeptical about the radiation that allows microwaves to heat items so quickly though...
Explanation:
This is common sense, mate. Sorry to break it to you.
which of the following are spontaneous processes? select all that apply: the conversion of rust to iron radioactive decay ice melting at room temperature and typical atmospheric pressure water flowing uphill
Answers
the conversion of rust to iron and water flowing uphill are not spontaneous, as they require external energy to reverse the natural direction of these processes.
The spontaneous processes among the given options are the conversion of rust to iron, radioactive decay, and ice melting at room temperature and typical atmospheric pressure. Water flowing uphill is not a spontaneous process as it violates the second law of thermodynamics. Spontaneous processes occur naturally without any external influence. Among the given options, radioactive decay and ice melting at room temperature and typical atmospheric pressure are spontaneous processes. Radioactive decay happens without any intervention, and ice melting occurs when the surrounding temperature is above its freezing point. However, the conversion of rust to iron and water flowing uphill are not spontaneous, as they require external energy to reverse the natural direction of these processes.
To know more about spontaneous visit:
https://brainly.com/question/5372689
#SPJ11
Answer: Ice melting and radioactive decay
a) Find the gas speed of sulfur dioxide at 100.0 degrees Celsius? ______________
b) What is the rate of effusion of sulfur dioxide compared to nitrogen triodide at the same temperature? __________________
Answers
a. 381.27 m/s
b. the rate of effusion of sulfur dioxide = 2.5 faster than nitrogen triiodide
Further explanationGiven
T = 100 + 273 = 373 K
Required
a. the gas speedi
b. The rate of effusion comparison
Solution
a.
Average velocities of gases can be expressed as root-mean-square averages. (V rms)
\(\large {\boxed {\bold {v_ {rms} = \sqrt {\dfrac {3RT} {Mm}}}}\)
R = gas constant, T = temperature, Mm = molar mass of the gas particles
From the question
R = 8,314 J / mol K
T = temperature
Mm = molar mass, kg / mol
Molar mass of Sulfur dioxide = 64 g/mol = 0.064 kg/mol
\(\tt v=\sqrt{\dfrac{3\times 8.314\times 373}{0.064} }\\\\v=381.27~m/s\)
b. the effusion rates of two gases = the square root of the inverse of their molar masses:
\(\rm \dfrac{r_1}{r_2}=\sqrt{\dfrac{M_2}{M_1} }\)
M₁ = molar mass sulfur dioxide = 64
M₂ = molar mass nitrogen triodide = 395
\(\tt \dfrac{r_1}{r_2}=\sqrt{\dfrac{395}{64} }=\dfrac{20}{8}=2.5\)
the rate of effusion of sulfur dioxide = 2.5 faster than nitrogen triodide
How many moles of iron(Il) oxide are produced from
3.0 moles of oxygen and excess Iron Sulfide as described by the chemical equation below?
4FeS + 502 - 2Fe,03 + 4502
Answers
Answer:
6 moles of Iron(II) Oxide
Answer:
1.2 mol
Explanation:
if the potential of a voltaic cell is 0.837 v, what is the free energy change when two moles of electrons are transferred in an oxidation-reduction reaction?
Answers
Gibb's free energy change is -1,61,541Joules when two moles of electrons are transferred in an oxidation-reduction reaction.
In thermodynamics, the Gibbs free energy (or Gibbs energy ) is a thermodynamic potential that can be utilized to compute the greatest measure of work that might be performed by a thermodynamically shut framework at consistent temperature and pressure. It likewise gives an important condition to cycles, for example, substance responses that might happen under these circumstances.
For calculating free energy change, we need to know about the value of faraday constant and voltage of the cell.
We know that 1 faraday=96500Coloumb,voltage=0.837,n=2
So, free energy(ΔG)= - n × F × E°
=>ΔG= -2 × 96500× 0.837
=>ΔG= -193000×0.837
=>ΔG= -1,61,541Joules (Here negative sign shows that voltaic cell is possible in this universe).
Hence, required free energy is -1,61,541Joules.
To know more about Gibb's free energy, visit here:
https://brainly.com/question/20358734
#SPJ4
explain, in terms of collision theory why an increase in temperature increases the rate of reaction between metheane gas and steam
Answers
Answer:
Hey
Explanation:
It is because the kinetic energy between particles increase and and the rate of effective collision between them increase so the rate of reaction increases.
show your work using dimensional analysis with the labels of atoms or molecules.
1.) 8.21 mol of P=
2.) 12.3 mol of Si=
3.) 0.93 mol of Mn=
Answers
Using dimensional analysis with the labels of atoms or molecules, we found that there are 4.94 * 10²⁴ atoms of P, 7.41 * 10²⁴ atoms of Si, and 5.60 * 10²³ atoms of Mn in the given moles.
Here's a step-by-step explanation for each problem:
1.) 8.21 mol of P:
Step 1: Identify the conversion factor. In this case, we will use the Avogadro's number, which is 6.022 * 10²³ atoms/mol.
Step 2: Apply dimensional analysis using the given moles of P and the conversion factor.
(8.21 mol P) * ( 6.022 * 10²³ atoms/mol) = 4.94 * 10²⁴ atoms of P.
2.) 12.3 mol of Si:
Step 1: Identify the conversion factor. In this case, we will use the Avogadro's number, which is 6.022 * 10²³atoms/mol.
Step 2: Apply dimensional analysis using the given moles of Si and the conversion factor.
(12.3 mol Si) * ( 6.022 * 10²³ atoms/mol) = 7.41 * 10²⁴ atoms of Si.
3.) 0.93 mol of Mn:
Step 1: Identify the conversion factor. In this case, we will use the Avogadro's number, which is 6.022 * 10²³ atoms/mol.
Step 2: Apply dimensional analysis using the given moles of Mn and the conversion factor.
(0.93 mol Mn) * ( 6.022 * 10²³ atoms/mol) = 5.60 * 10²³ atoms of Mn.
learn more about Avogadro's number Refer: https://brainly.com/question/28812626
#SPJ11
Like any other equilibrium constant, kw is also affected by temperature. the kw at 75 degrees celsius is 1.995 x 10⁻¹³. What is the poh of water at this temperature?
Answers
Like any other equilibrium constant, kw is also affected by temperature. the kw at 75 degrees Celsius is 1.995 x 10⁻¹³. Therefore, 13 is the pOH of water at this temperature.
What is pOH?The concentration of hydroxide ions (OH-) is measured by pOH. It is utilized to express a solution's alkalinity.
Aqueous solutions having pOH less than 7 at 25 degrees Celsius are alkaline, pOH larger than 7 are acidic, while pOH equal with 7 are neutral. pH and hydrogen ion concentration is used to compute pOH.
Kw = [OH⁻] [H⁺]
1.995 x 10⁻¹³ = Kw
[OH⁻] =[H⁺]
Kw = [OH⁻]²
[OH⁻] =10⁻¹³
pOH = -log [OH⁻]
= - log 10⁻¹³
= 13
Therefore, 13 is the pOH of water at this temperature.
To know more about pOH, here:
https://brainly.com/question/4092108
#SPJ1
the molecular orbital model of bonding states that when two atomic orbitals combine, they will form two molecular orbitals, one
Answers
When two atomic orbitals combine, they will form two molecular orbitals, one bonding and one antibonding.
The molecular orbital model of bonding describes the behavior of electrons in molecules by considering the overlap of atomic orbitals. When two atomic orbitals come together, they can either constructively combine to form a bonding molecular orbital or destructively combine to form an antibonding molecular orbital.
In the bonding molecular orbital, the wave functions of the atomic orbitals add up in-phase, leading to a region of electron density between the nuclei. This bonding orbital stabilizes the molecule and promotes electron sharing between the atoms, resulting in a stronger bond.
On the other hand, in the antibonding molecular orbital, the wave functions of the atomic orbitals add up out-of-phase, resulting in a region of electron density away from the nuclei. This antibonding orbital does not contribute to the stability of the molecule and can weaken or even break the bond.
The molecular orbital model accounts for the concept of electron delocalization and provides insights into the electronic structure and properties of molecules. By considering the formation of both bonding and antibonding molecular orbitals, it allows us to understand the strength of chemical bonds and predict the reactivity of molecules.
Learn more about the molecular orbital model and its implications in understanding chemical bonding and molecular properties.
Learn more about molecular orbital
brainly.com/question/29642622
#SPJ11
Q.
.No.of valence electrons present in 4.2g of nitride ion (N^(3- are:
(a) 2.4times N
(b) 4.2times N
(c) 1.6times N
(d) 3.2times N
Answers
Answer:
if na is Avogadros number then number of vaclence electron in 4.2 g of nitride ions (N3-is.2.4Na. so the answer is 2.4 na
Explanation:
Mass N3- ion = 14 g/mol. So,
4.2 g X (1 mol/14 g) = 0.3 mol N3-
Number of N3- ions = 0.3 mol X 6.02 X 10^23 ions/mol = 1.8 X 10^23 ions
Each N3- ion has 8 valence electrons, so total number of valence electrons = 1.8 X 10^23 X 8 = 1.4 X 10^24 valence electrons.
hope it helps
Which of the following is the conjugate acid of H2PO4−?
A.
H3PO4
B.
H4PO4−
C.
HPO4−2
D.
PO43−
Answers
The conjugate acid of \(H_2PO_4\)− is option a) \(H_3PO_4.\)
The terms in this answer are "conjugate acid" and "\(H_2PO_4\).What is a conjugate acid?A conjugate acid is an acid that creates another acid by accepting a proton (H+ ion).Consider the following reaction:HF +\(H_2O\) ⇌ \(H_3O\)+ + F-The acid HF and the base F- are two conjugate acid-base pairs.
When the acid HF donates a proton (H+ ion), the base F- becomes the conjugate acid because it accepts the proton (H+ ion). \(H_3O\)+ is the conjugate acid of \(H_2O\) because it gains a proton (H+ ion) from water.Conjugate base is a species formed when an acid donates a proton. In comparison to a particular base, it has one less proton.
On the other hand, the acid and base pairs are known as a conjugate acid-base pair.In the provided options, \(H_3PO_4.\) is the only compound which can gain a proton to form the conjugate acid. Therefore, the correct option is A. \(H_3PO_4.\)
Know more about conjugate acid here:
https://brainly.com/question/30225097
#SPJ8
4- Which of the following is not a property of vinegar?
(a) Dissolve on water gives H*
(b) Has a slippery feel
(C) Turns litmus from blue to red
(d) React with zinc gives hydrogen gas.
Answers
Answer:
The correct answer is (b)
Explanation:
Has a slippery feel.Vinegar is an aqueous solution of acetic acid, and it has a number of properties that make it useful in a variety of applications. Some of these properties include:(a) Dissolves in water to give an aqueous solution of acetic acid (H*): Vinegar is composed of water and acetic acid, and it can be dissolved in water to form an aqueous solution.(c) Turns litmus from blue to red: Litmus is a type of dye that is used as an indicator in chemical reactions. It turns blue in the presence of an alkaline solution and red in the presence of an acidic solution. When vinegar is added to litmus, it will turn the litmus red, indicating that it is an acidic solution.(d) Reacts with zinc to give hydrogen gas: When vinegar is mixed with zinc, it can produce hydrogen gas. This reaction is an example of a redox reaction, in which the vinegar is reduced (gains electrons) and the zinc is oxidized (loses electrons).However, vinegar does not have a slippery feel, so (b) Has a slippery feel is not a property of vinegar.
How does a heat affect the chemical reaction?
Answers
Answer:
Increasing the temperature increases reaction rates because of the disproportionately large increase in the number of high energy collisions. It is only these collisions (possessing at least the activation energy for the reaction) which result in a reaction.
Explanation:
Calculate the volume of oxygen required at stp for complete combustion of 2.3g of ethanol the reaction is
Answers
Taking into account the reaction stoichiometry and STP conditions, the volume of O₂ required at STP for complete combustion of 2.3g of ethanol is 3.36 L.
Reaction stoichiometryThe balanced reaction is:
CH₃CH₂OH + 3 O₂ → 2 CO₂ + 3 H₂O
By reaction stoichiometry, the following amounts of moles of each compound participate in the reaction:
CH₃CH₂OH: 1 moleO₂: 3 moles CO₂: 2 molesH₂O: 3 molesThe molar mass of the compounds is:
CH₃CH₂OH: 46 g/moleO₂: 32 g/moleCO₂: 44 g/moleH₂O: 18 g/moleBy reaction stoichiometry, the following mass quantities of each compound participate in the reaction:
CH₃CH₂OH: 1 mole× 46 g/mole= 46 gramsO₂: 3 moles ×32 g/mole= 96 gramsCO₂: 2 moles ×44 g/mole= 88 gramsH₂O: 3 moles ×18 g/mole= 54 gramsSTP conditionsThe STP conditions refer to the standard temperature and pressure. The values are 0 °C and 1 atmosphere and are reference values for gases. In these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
Moles of O₂ requiredIt can be applied the following rule of three: if by reaction stoichiometry 46 grams of ethanol react with 3 moles of O₂, 2.3 grams of ethanol react with how many moles of O₂?
moles of O₂= (2.3 grams of ethanol× 3 moles of O₂)÷ 46 grams of ethanol
moles of O₂= 0.15 moles
Then, 0.15 moles of O₂ react.
Volume of NO createdNow, you can apply the following rule of three: if by definition of STP conditions 1 mole of O₂ occupies a volume of 22.4 liters, 0.15 moles occupies how much volume?
volume= (0.15 moles× 22.4 L)÷ 1 mole
volume= 3.36 L
Finally, the volume of O₂ is 3.36 L.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
#SPJ1
Choose all the answers that apply. lonic compounds ___.
A. do not dissolve in water
B. have high melting points
C. have low melting points
D.dissolve easily in water
E. conduct electricity when melted
(science class not chemistry)
Answers
Answer:
E conduct electricity when melted
Explanation:
They can not produce electricity until dissolved/ melted in water
The cultures of prehistoric humans are known mostly through the excavation of stone tools and other relatively imperishable artifacts. The early tool making traditions are often referred to as being paleolithic (literally "Old Stone Age). The Oldowan and Acheulian tool traditions of the first humans were the simplest applied research basic research Scientihe thought O philosophies technologies
Answers
The cultures of prehistoric humans are primarily known through the excavation of stone tools and other durable artifacts, such as the Oldowan and Acheulian tool traditions.
Stone tools and imperishable artifacts serve as key archaeological evidence for understanding prehistoric cultures. Through meticulous excavation and analysis, archaeologists have been able to piece together the lifestyles, technological advancements, and social behaviors of early human societies. The term "paleolithic" refers to the Old Stone Age, a time when humans relied on stone tools as their primary implements.
The Oldowan tool tradition is considered the earliest stone tool industry, dating back around 2.6 million years ago. It is characterized by simple tools, such as choppers and scrapers, which were crafted by flaking off pieces from larger stones. These tools were primarily used for basic activities like butchering and processing animal carcasses.
Later, the Acheulian tool tradition emerged around 1.76 million years ago, representing an advancement in stone tool technology. Acheulian tools, such as handaxes and cleavers, were more refined and standardized, showcasing an increased level of sophistication in tool-making techniques. These tools served a wide range of purposes, including hunting, woodworking, and shaping raw materials.
By studying the Oldowan and Acheulian tool traditions, researchers gain valuable insights into the cognitive abilities, cultural development, and technological progress of early humans. The examination of these artifacts provides evidence of their adaptability, problem-solving skills, and the gradual refinement of their tool-making techniques over time.
Learn more about prehistoric humans
brainly.com/question/28301954
#SPJ11
Why are noble gases called "safe" gases?
Answers
Answer:
Noble gases are called "safe" gases because they are inert.
General Formulas and Concepts:
Reading a Periodic TableChemical Properties of Noble GasesExplanation:
Because Noble Gases have filled their outer shell electrons fully, they tend not to form compounds with other elements or react with others to produce another compound.
Take Ne (neon) for instance. It's electron configuration is 1s²2s²2p⁶. The outer most shell is the 2p sublevel, and according quantum numbers, the 2p sublevel only has 3 spaces for 2 electrons each that it can hold. Therefore, Ne has a full outer shell and no electron spots to create a bond with other elements.
During an investigation, a scientist heated 123.6 g of copper carbonate till it decomposed to form a black residue. The total mass of the black residue formed was 79.6 g. Does the law of conservation of mass hold true in this case?
Answers
No, the Law of Conservation of Mass does not hold true for this case because the mass of reactant is not equal to the mass of product.
What is Law of Conservation of Mass?
According to the Law of Conservation of Mass, during a chemical reaction, the total masses of the products and reactants must be equal.This law states that although mass can be reorganized in space or the entities connected to it can change shape, mass itself cannot be generated or destroyed.For instance, the mass of the components prior to a chemical reaction (reactants) is equal to the mass of the components following the reaction (products).Therefore, the entire mass of the reactants, or starting materials, must be equal to the mass of the products during any chemical reaction and low-energy thermodynamic processes in an isolated system.Here, the mass of the reactant (copper carbonate) is not equal to the mass of the product (black residue). Hence, the Law of Conservation of Mass is not applicable in this case.To learn more about Law of Conservation of Mass from the given link
https://brainly.com/question/15289631
#SPJ1
What is the PRECAUTION for an Irritant?
Answers
Can someone help me here please i need this :(
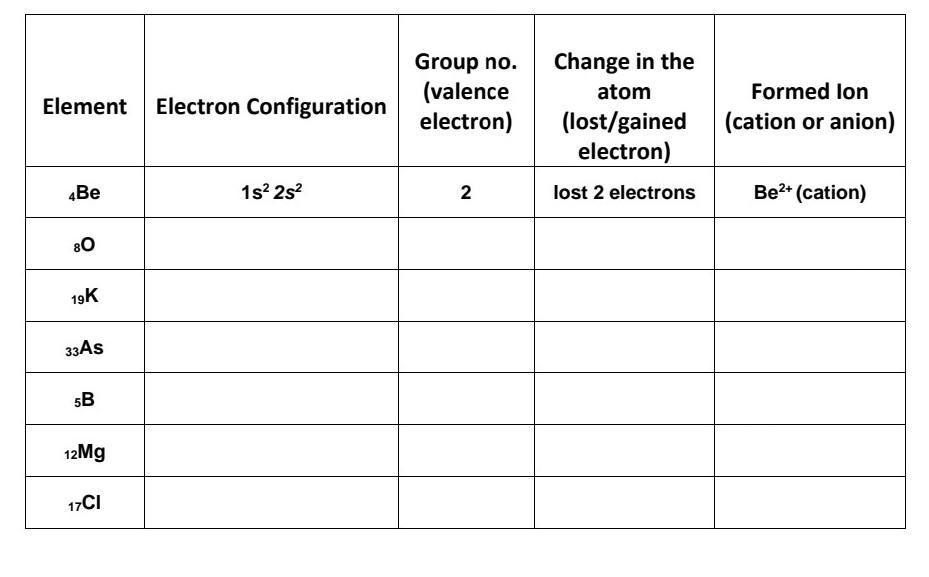
Answers
If 2 carbon atoms, underwent fusion. What element would you get?
Answers
Answer:
Two Helium atoms
If two carbon atoms underwent fusion (of 600 million kelvin) you would get two helium atoms.
An organic compound that is distilled from wood has a molar mass of 32.04 g/mol. its composition by mass is 37.5% carbon, 12.6% hydrogen, and 49.9% oxygen. Draw the Lewis structure of this compound.
Answers
The compound has SP3 hybridization and total of 14 valence electrons in its shell. we found this from the Lewis structure.
Distilled from wood Molar mass of organic compound is 32.04g/mole.
molar mass of carbon is 12 g/mole.
molar mass of hydrogen is 1g/mole.
molar mass of oxygen is 16g/mole.
The composition of organic compound by its mass is 37.5% carbon, 12.6% hydrogen, and 49.9% oxygen.
so, we can calculate the number of mole.
so, number of moles of C is, 37.5/ 12 = 3.13
number of moles of H is 12.6 / 1 = 12.6
number of moles of O is 49.9/ 16= 3.12
The molar ratio of carbon, hydrogen and oxygen is ,
C : H : O = 3.13 : 12.6 : 3.12
=1 : 4 : 1
So from this we found the empirical formula. The empirical formula of compound is CH4O.
valence electron of c is, 4.
valence electron of H is 4
valence electron of O is 6.
so the total valence electron is 14.
Form this we can draw the Lewis structure. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. This have SP3 hybridization.
To learn more about Lewis structure please visit:
https://brainly.com/question/20300458
#SPJ4
which instrument would a scientist use to measure out an exact mass of a dry chemical?
Answers
A scientist would use a balance to measure out an exact mass of a dry chemical. A balance is a laboratory instrument that measures mass by comparing a known mass with an unknown mass.
The balance uses a beam with a fulcrum and pans on both sides. The unknown mass is placed on one side of the balance, and known masses are added to the other side until the beam is balanced.
Once the balance is balanced, the mass of the unknown can be determined. This method of measuring mass is highly accurate, making it ideal for scientific experiments that require precise measurements.
To ensure accuracy, the balance should be calibrated regularly and used in a controlled environment to avoid any external factors that may affect the measurements.
To know more about scientist. please visit.....
brainly.com/question/2102672
#SPJ11
the question is on the screenshot worth 20 points pls help

Answers
Answer:
Cell Y Only
Explanation:
In the following reaction:-Assign the oxidation number.-Identify the oxidizing and reducing agent.-Identify the change in oxidation number.P4 + 10 Cl2 —> 4 PCl5
Answers
As you already know, P is being oxidized and it is the reducing agent in this reaction, as Chlorine is being reduced and it is the oxidizing agent for the reaction, but for oxidation number, there is one crucial information that will give us the answer:
Oxidation number for halogens will 99% of the times be -1, since they have 7 electrons in the valence shell and they need 1 to stabilize.
Cl2 is stable as the 2 chlorines are sharing electrons, but Cl alone will have a -1 charge, and Cl with other elements making up a compound will also have a -1 charge
Therefore if we have 5 Chlorines with P, and each Chlorine has a -1 charge, therefore Phosphorus will have a +5 charge in order to balance and stabilize charges, and this is experimentally proven, as P has oxidation numbers = -3, +3 and +5.
Oxidation number change
P = P^5+
Cl = Cl^-
estimate the anharmonicity constant for 1^H^81Br. What is the absorption wavenumber of the first overtone of H^19F?
Answers
The potential energy surface (PES) of a molecule is conceptualised in quantum mechanics as a harmonic oscillator with evenly spaced energy levels for vibrational modes. In fact, the PES is more intricate and exhibits anharmonicity, which means that the vibrational modes' energy levels are not evenly distributed.
An anharmonicity constant, which measures the PES's departure from a harmonic oscillator, can be used to define anharmonicity.
It is possible to calculate the absorption wavenumber of H19F's first overtone using the following formula:
v = 2ν_0 - ν_1
where v_0 denotes the basic absorption wavenumber and _1 the first overtone wavenumber. The initial overtone's wavenumber is typically around twice as large as the fundamental. The first overtone's wavenumber can be calculated to be approximately 5680 cm-1 for H19F since the fundamental absorption wavenumber is around 2840 cm-1. It's crucial to remember that this is merely an estimate, and the actual value may change based on the particular molecule and the experimental setup.
In conclusion, even though I am unable to estimate the anharmonicity constant for 1H81Br precisely, I can give some general information about anharmonicity and how it relates to vibrational spectra. A straightforward formula was also used to calculate the absorption wavenumber of the first overtone of H19F.
For more such question on potential
https://brainly.com/question/24501251
#SPJ11
To estimate the anharmonicity constant for 1^H^81Br, we can use the equation:
ν = 2ν₁ - ν₂
where ν is the frequency of the first overtone, ν₁ is the frequency of the fundamental vibration, and ν₂ is the frequency of the second overtone. We can assume that the fundamental frequency is equal to the experimental frequency of the absorption peak and that the second overtone frequency is equal to 3 times the fundamental frequency.
For 1^H^81Br, let's assume that the fundamental frequency is 3000 cm^-1. Then the frequency of the second overtone would be 9000 cm^-1. Using the equation above, we can calculate the frequency of the first overtone:
ν = 2(3000 cm^-1) - 9000 cm^-1 = -3000 cm^-1
Note that this value is negative, which indicates that the anharmonicity constant for 1^H^81Br is likely quite large.
For the absorption wavenumber of the first overtone of H^19F, we need to know the fundamental frequency of the molecule. Let's assume that the fundamental frequency of H^19F is 4000 cm^-1. Then the frequency of the first overtone would be:
ν = 2(4000 cm^-1) - 4000 cm^-1 = 4000 cm^-1
Converting this to wavenumber gives:
4000 cm^-1 / (1 cm^-1) = 4000 cm^-1
Therefore, the absorption wavenumber of the first overtone of H^19F is estimated to be 4000 cm^-1.
Learn more about anharmonicity here:
https://brainly.com/question/31424950
#SPJ11