3. how many moles are present in 10.0 g of aluminum(Al)
Answers
Answer:
1 Mole
here is answer
hope it will help you
Related Questions
Which describes an element?
They cannot be broken down any further.
They can combine with other elements to form atoms.
They were all discovered at the same time.
They were created in the Earth’s core.
Answers
Answer:
They cannot be broken down any further
Explanation:
An element is a substance that cannot be broken down into a simpler format. They are distinguished by a unique atomic number. The elements are organized by their atomic number in the periodic table, which highlights elements with similar properties.
4 Al + 3 O2 -> 2 Al2O3 How much aluminum would be needed to completely react with 45 grams of Oz?
Answers
Answer:
50.6g Al would be needed
Explanation:
Based on the reaction:
4Al + 3O₂ → 2Al₂O₃
4 moles of aluminium react with 3 moles of oxygen to produce 2 moles of Al₂O₃
To solve this question we need to find the moles of 45g of O₂. Then using the chemical equation find the moles of Al and its mass:
Moles O₂ -Molar mass: 32g/mol-:
45g O₂ * (1mol / 32g) = 1.41 moles O₂
Moles Al:
1.41 moles O₂ * (4mol Al / 3mol O₂) = 1.875 moles Al
Mass Al -Molar mass: 26.98g/mol-:
1.875 moles Al * (26.98g / mol) =
50.6g Al would be needed
The amount of aluminum that would be required to completely react with 45 grams of O2 would be 50.59 grams.
From the balanced equation of the reaction:
\(4 Al + 3 O_2 ---> 2 Al_2O_3\)
The mole ratio of Al to O2 is 4:3.
Mole of 45 grams of O2 = mass/molar mass
= 45/32
= 1.4063 moles
Equivalent mole of Al: 4 x 1.4063/3
= 1.875 moles
mass of Al = mole x molar mass
= 1.875 x 26.98
= 50.59 grams
More on stoichiometry calculations can be found here: https://brainly.com/question/22288091?referrer=searchResults
If the half-life of a radioactive substances is 590 million years and you have 40 atoms of it, how many half-lives will have passed when 5 atoms remain
Answers
Answer:
3
Explanation:
Applying,
\(2^{n'}\) = R/R'............... Equation 1
Where n' = number of halflives that have passed, R = Original atom of the substance, R' = atom of the substance left after decay.
From the question,
Given: R = 40 atoms, R' = 5 atoms
Substitute these values into equation 1
\(2^{n'}\) = 40/5
\(2^{n'}\) = 8
\(2^{n'}\) = 2³
Equation the base,
n' = 3
A lump of zinc is tossed into a beaker of 500L of 14M hydrochloric acid. this reaction produces Hydrogen Gas and zinc (II) chloride. If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, what is the mass of the zinc?
Answers
If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, 2796.96 g mass of the zinc is produced .
Using the ideal gas law equation:
PV = nRT
n = (PV) / (RT)
= (1.75 atm * 645 L) / (0.0821 atm·L/(mol·K) * 400 K)
= 42.71 moles
the balanced equation for the reaction between zinc and hydrochloric acid:
Zn + 2HCl -> \(ZnCl_{2}\) + \(H_{2}\)
1 mole of zinc produces 1 mole of hydrogen gas. Therefore, the moles of zinc are also 42.71.
The molar mass of zinc is 65.38 g/mol.
Mass of zinc = moles of zinc * molar mass of zinc
= 42.71 moles * 65.38 g/mol
= 2796.96 g
Therefore, the mass of the zinc is 2796.96 grams.
learn more about hydrogen gas :
https://brainly.com/question/30829657
During the process of Photosynthesis, light/ radiant energy is transformed in to chemical energy.
solar
energy
Photosynthesis
6C0, + 6H20
C.H,206 + 602
glucose oxygen
water
carbon
dioxide
True
O False
Answers
photosynthesis uses light from the sun to create glucose (a chemical) that the plant uses for energy. the plant transforms light energy into chemical energy
Explain why a climax community is not always a forest.
Answers
In the reaction
Cu° + 2 H2SO4 → CuSO4 + 2 H2O + SO2,
copper is
A) reduced and is the oxidizing agent
B) reduced and is the reducing agent
C) oxidized and is the oxidizing agent
D) oxidized and is the reducing agent
Answers
EXPLANATION: Copper goes from a charge of 0 in Cu to +2 in CuSO4 so it is oxidized. A reducing agent is the substance being oxidized.
Computer architecture. Can you
please answer all the question. True or false, Thank you
1. Indicate whether the following statements are true of false. ( ) In the same OSM, all pages must have the same size. ( ) In the same OSM, all segments must have the same size. ) Internal fragmentat
Answers
a. False. In the same OSM (Operating System Memory), it is not necessary for all pages to have the same size. Different pages can have varying sizes depending on the memory allocation requirements of the system.
b. False. In the same OSM, all segments do not need to have the same size.
Segments can have different sizes based on the specific needs of the programs or processes running in the system.
Segmentation allows for flexible memory allocation by dividing the memory into logical segments.
c. True. Internal fragmentation occurs when memory is divided into smaller areas to accommodate smaller data blocks, but the remaining memory within each block is too small to be utilized for another block.
As a result, these small unused portions of memory remain empty, leading to inefficient memory utilization.
This is a disadvantage of the paging architecture employed in a computer's memory management system. It can result in wasted memory space and lower overall system performance.
Read more about Operating System Memory
https://brainly.com/question/30593994
#SPJ11
how many grams of phosphorus are in 50-gram sample of aluminum phosphate
Answers
There are approximately 12.7 grams of phosphorus in a 50-gram sample of aluminum phosphate.
To determine the number of grams of phosphorus in a 50-gram sample of aluminum phosphate, we need to know the molar mass and the chemical formula of aluminum phosphate.
The chemical formula for aluminum phosphate is AlPO4. It indicates that each molecule of aluminum phosphate contains one aluminum atom (Al), one phosphorus atom (P), and four oxygen atoms (O).
To calculate the molar mass of aluminum phosphate, we can add up the atomic masses of its constituent elements based on their stoichiometric ratios:
Molar mass of AlPO4 = (molar mass of Al) + (molar mass of P) + (4 * molar mass of O)
Using the periodic table, we can find the atomic masses of the elements:
Molar mass of Al = 26.98 g/mol
Molar mass of P = 30.97 g/mol
Molar mass of O = 16.00 g/mol
Now, let's calculate the molar mass of aluminum phosphate:
Molar mass of AlPO4 = (26.98 g/mol) + (30.97 g/mol) + (4 * 16.00 g/mol)
= 121.95 g/mol
The molar mass of aluminum phosphate is 121.95 g/mol.
To determine the number of grams of phosphorus in a 50-gram sample of aluminum phosphate, we need to calculate the mass fraction of phosphorus in the compound. The mass fraction is the ratio of the molar mass of phosphorus to the molar mass of aluminum phosphate.
Mass fraction of phosphorus = (molar mass of P) / (molar mass of AlPO4)
= (30.97 g/mol) / (121.95 g/mol)
≈ 0.254
Multiplying the mass fraction by the mass of the sample gives us the grams of phosphorus:
Grams of phosphorus = (mass fraction of phosphorus) * (mass of the sample)
= 0.254 * 50 g
≈ 12.7 g
For more such questions on aluminum phosphate visit:
https://brainly.com/question/15072110
#SPJ8
Which of the following describes freezing?
A substance changing from the liquid to the gaseous state.
A substance changing from the gaseous state to the liquid state.
A substance changing from the liquid state to the solid state.
A substance changing from the solid state to the liquid state.
Answers
Answer:
C. a substance changing from the liquid state to the solid state.
Explanation:
Answer:
C
Explanation:
C
Is hydrogen positive or negative charge?
Answers
Hydrogen is said to be positively charged and not negatively charged.
How is Hydrogen positively charged?A hydrogen atom loses an electron, becoming positively charged (it has a charge of +1), and this creates a hydrogen ion. Due to the fact that a hydrogen atom only possesses one proton and no electrons, a hydrogen atom is frequently referred to as only a proton.
A proton in the nucleus of a hydrogen atom is surrounded by one electron. In order to form a full outer shell and become more stable, hydrogen usually loses this electron or shares it with another atom in a covalent bond. Hydrogen is only one proton thick when its electron is removed, giving it a positive charge.
Read more on Hydrogen here:https://brainly.com/question/24433860
#SPJ1
What is the name of this molecule?

Answers
Answer:
We are to name the compound:
Assuming carbon is represented by the big gray atoms while the smaller ones represents hydrogen atoms and the the red atom represents oxygen, the chemical formula of the compound is:
CH3CH2CH2OH
draw the structures and give the names of the simplest straight-chain (the triple bond between c1 – c2) alkynes containing seven to twelve carbons.
Answers
The simplest straight-chain alkynes containing seven to twelve carbons are:
1. Heptyne (7 carbons)
2. Octyne (8 carbons)
3. Nonyne (9 carbons)
4. Decyne (10 carbons)
5. Undecyne (11 carbons)
6. Dodecyne (12 carbons)
Here are the structures and names of the simplest straight-chain alkynes containing seven to twelve carbons:
1. Heptyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Heptyne
2. Octyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Octyne
3. Nonyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Nonyne
4. Decyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Decyne
5. Undecyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Undecyne
6. Dodecyne:
Structure: H-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-C≡C-H
Name: 1-Dodecyne
Learn more about alkynes at https://brainly.com/question/27937735
#SPJ11
Which two geologic events occur very slowly as a result of tectonic forces and movement?
A.Convection in the mantle
B.Earthquakes
C.Plate subduction
D.Formation of volcanoes
Answers
The two geologic events which occur very slowly as a result of tectonic forces and movement are convection in the mantle and plate subduction.
What is Subduction?This is a term which is referred to as the process in which there is collision between two of Earth's tectonic plates, where one plate sinks into the mantle underneath the other plate.
This is caused by geologic events which occur very slowly as a result of tectonic forces while on the other hand, mountains and volcanoes occur abruptly which is therefore the reason why options A and C were chosen as the correct choice.
Read more about Subduction here https://brainly.com/question/1358208
#SPJ1
Answer: A and C
Explanation:
Use the value of the activation energy (Ea = 1.50 x 102 kJ/mol) and the given rate constant of the reaction at either of the two temperatures to predict the rate constant at 542 K. The rate constant at 701 K is measured as 2.57 M−1⋅s−1 and that at 895 K is measured as 567 M−1⋅s−1.
Answers
To predict the rate constant at 542 K using the given activation energy and the rate constant at 701 K, we can apply the Arrhenius equation. By substituting the values into the equation, we can calculate the rate constant at the desired temperature.
To predict the rate constant at 542 K using the given activation energy (Ea = 1.50 x 10^2 kJ/mol), we can use the Arrhenius equation:
k2 = k1 * e^(-Ea / (R * T2))
where k1 is the rate constant at the known temperature (701 K), k2 is the rate constant at the desired temperature (542 K), Ea is the activation energy, R is the gas constant (8.314 J/(mol*K)), and T2 is the temperature in Kelvin (542 K).
First, we need to convert the activation energy from kJ/mol to J/mol by multiplying it by 1000:
Ea = 1.50 x 10^2 kJ/mol * 1000 J/1 kJ = 1.50 x 10^5 J/mol
Now, we can calculate the rate constant at 542 K:
k2 = 2.57 M^(-1)s^(-1) * exp(-1.50 x 10^5 J/mol / (8.314 J/(molK) * 542 K))
k2 ≈ 2.57 M^(-1)*s^(-1) * exp(-34.84)
Using the above calculation, we can determine the rate constant at 542 K.
For more such question on energy. visit :
https://brainly.com/question/29339318
#SPJ8
how many CO2 molecules exist in the following reaction? C3H8 + 5O2 >>> 3CO2 + 4H2O
Answers
Answer:
21.0g of CO2
Explanation:
can someone help me solve the questions below using the data table below PLEASEE
DATA TABLE:
Mass of flask and vinegar solution- 25.17g
Mass of flask- 15.12g
Volume of vinegar solution (in mL)- 10.00ml
Initial volume of NaOH (in mL)-0.00ml
Final volume of NaOH (in mL)-39.00ml
CALCULATIONS:
Mass of vinegar solution- 10.0503g
Volume of NaOH used in titration (in mL)-39.00ml

Answers
The molarity of NaOH solution is 0.114 M.
Given, Mass of flask and vinegar solution= 25.17 g.Mass of flask= 15.12 gVolume of vinegar solution (in mL)= 10.00 mlInitial volume of NaOH (in mL)= 0.00 mlFinal volume of NaOH (in mL)= 39.00 mlThe Mass of vinegar solution is 10.0503 g.The volume of NaOH used in titration is 39.00 ml.Let's calculate the molarity of the NaOH solution.First, calculate the moles of NaOH used in the reaction. Moles of NaOH = Molarity × Volume of NaOH (in L) Converting volume in mL to L,Volume of NaOH used = 39.00 mL = 39.00/1000 L = 0.0390 LThe molarity of NaOH solution is given by;Molarity of NaOH = Moles of NaOH / Volume of vinegar solution (in L)Converting volume in mL to L,Volume of vinegar solution = 10.00 mL = 10.00/1000 L = 0.0100 LNow, substituting the values; Molarity of NaOH = 0.114 M.
for such more questions on molarity
https://brainly.com/question/30404105
#SPJ8
Give an example of how knowledge of physical properties of matter can be used in everyday life
Answers
Understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
Knowledge of physical properties of matter is extremely important in everyday life as it helps us understand the nature of substances we come into contact with. One example is the use of boiling points in cooking. Different substances have different boiling points which determine the temperature at which they boil. This information is crucial in determining cooking times and ensuring that food is cooked properly.
For instance, water boils at 100 degrees Celsius, while sugar syrup boils at a much higher temperature. If the wrong temperature is used, food may be undercooked or overcooked, leading to undesired outcomes. Knowledge of physical properties also helps in choosing the right materials for different purposes, such as choosing heat-resistant materials for cooking.
In conclusion, understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
To know more about matter visit:
brainly.com/question/28487167
#SPJ11
How many moles are contained in 50. 0L of C2H6 gae at STP
Answers
There are 2.23 moles of C2H6 gas in 50.0L at STP. To determine the number of moles in a given volume of gas at STP, we can use the ideal gas law equation.
The ideal gas law equation is PV = nRT, where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant, and T is temperature. At STP, the pressure is 1 atm and the temperature is 273.15 K. So the calculation is
PV = nRT
n = \(\frac{P.V}{R.T}\)
n = \(\frac{(1 atm) (50 L)}{(0.0821 L.atm/mol.K)(273.15K)}\)
n = 2.23 mol
Learn more about the ideal gas equation: https://brainly.com/question/29796637
#SPJ11
after the child exhaled all of the gas, it becomes sick
Answers
how many electrons does Co2- have?
Answers
Answer:
Explanation:
6 electrons
carbon dioxide, C has atomic number 6 so it has 6 electrons and oxygen has atomic no 8, i.e 16 electrons.
what is the valency of water
Answers
Answer:
In the water molecule oxygen combines with two hydrogens and so has a valency of 2. A simple diagram of a water molecule makes this plain. The hydrogen atoms are each sharing one pair of electrons - they have a valency of 1. The oxygen atom is sharing two pairs of electrons - it has a valency of 2.
Which of the following acids will not dissociate completely in water? Pick only one. HCl HClO4 HClO HNO3
Answers
HClO will not dissociate completely in water among the given option.
When acids dissolve in water, they can dissociate into ions. Strong acids dissociate completely, while weak acids only partially dissociate. To determine which acid will not dissociate completely, we need to identify the weak acid among the options.
HClO is a weak acid known as hypochlorous acid. It does not dissociate completely in water. Instead, it partially dissociates into H⁺ and ClO⁻ ions.
On the other hand, HCl, HClO₄, and HNO₃ are strong acids and dissociate completely in water, producing H⁺ ions. These strong acids are considered to be fully ionized in aqueous solutions.
learn more about acids here:
https://brainly.com/question/29796621
#SPJ11
are the bonds in each of the following substances ionic, nonpolar covalent, or polar covalent?
Answers
Answer:
no there is no bonds between the two of these
can some give 2 paragraph about science
Answers
Answer:
Explanation:Science is the scholarly and down to earth movement, including the efficient investigation of the structure and the laws of nature with intensive trial and pragmatic perception. Science is isolated into three branches: Natural sciences, Social sciences, formal sciences.
Science offers new information and revelations of many things we do not know. Day by day, science is progressing. Now science has helped man reach the moon, which was once unknown to man as a satellite. Every invention in technology or medical techniques is based on science and scientific research
Ionic compounds have a net charge of what?
Answers
Ionic compounds have a net charge of what ?
zero
Any ionic compound will have a net charge of zero. Another way of saying this is that cations and anions must always combine in such a way so that their charges cancel.
Answer:zero
Any ionic compound will have a net charge of zero. Another way of saying this is that cations and anions must always combine in such a way so that their charges cancel.
Explanation:
11) A sample of gas has a volume of 823.7 mL and a pressure of 351.8 torr. What volume (in mL) will the gas occupy at 752.9 torr if the temperature of the gas doesn't change? Keep the answer with one decimal place
Answers
Answer:
384.9 mL.
Explanation:
What is given?
Volume 1 (V1) = 823.7 mL.
Pressure 1 (P1) = 351.8 torr.
Pressure 2 (P2) = 752.9 torr.
What do we need? Volume 2 (V2).
Step-by-step solution:
This is a Boyle's Law problem. Boyle's law states that the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant. An inverse relationship is described in this way. As one variable increases in value, the other variable decreases.
The formula of the law is:
\(P_1V_1=P_2V_2.\)Where P is pressure and V is volume. We want to find volume 2, 'V2'. So let's solve for 'V2' and replace the given data that we have in the new formula:
\(V_2=\frac{P_1V_1}{P_2}=\frac{351.8\text{ torr}\cdot823.7\text{ mL}}{752.9\text{ torr}}=384.882\text{ mL}\approx384.9\text{ mL.}\)The new volumewill be 3384.9 mL You can note as the pressure is increasing, the volume is decreasing.
- What is the change in enthalpy when 36.00 g of aluminum reacts with excess ammonium nitrate
(NH4NO3) according to the equation: (5 points)
2A1+ 3NH4NO3 → 3N2 + 6 H₂O + Al2O3 AH = -2030kJ
Answers
The molar mass of aluminum (Al) is 26.98 g/mol.
First, we need to calculate the moles of aluminum (Al) in 36.00 g of aluminum:
moles of Al = mass of Al / molar mass of Al
moles of Al = 36.00 g / 26.98 g/mol ≈ 1.334 mol
From the balanced equation, we can see that the stoichiometric ratio between aluminum (Al) and the change in enthalpy is 2: -2030 kJ. This means that for every 2 moles of aluminum reacting, the change in enthalpy is -2030 kJ.
Next, we can use the stoichiometry to calculate the change in enthalpy for the given amount of aluminum:
change in enthalpy = moles of Al * (change in enthalpy / stoichiometric coefficient of Al)
change in enthalpy = 1.334 mol * (-2030 kJ / 2) ≈ -1362.68 kJ
Therefore, the change in enthalpy when 36.00 g of aluminum reacts with excess ammonium nitrate (NH4NO3) is approximately -1362.68 kJ.
I need help with both of the questions fast plz!!!

Answers
Answer: SMALLEST is lithium
LARGEST is Fluorine
Explanation:
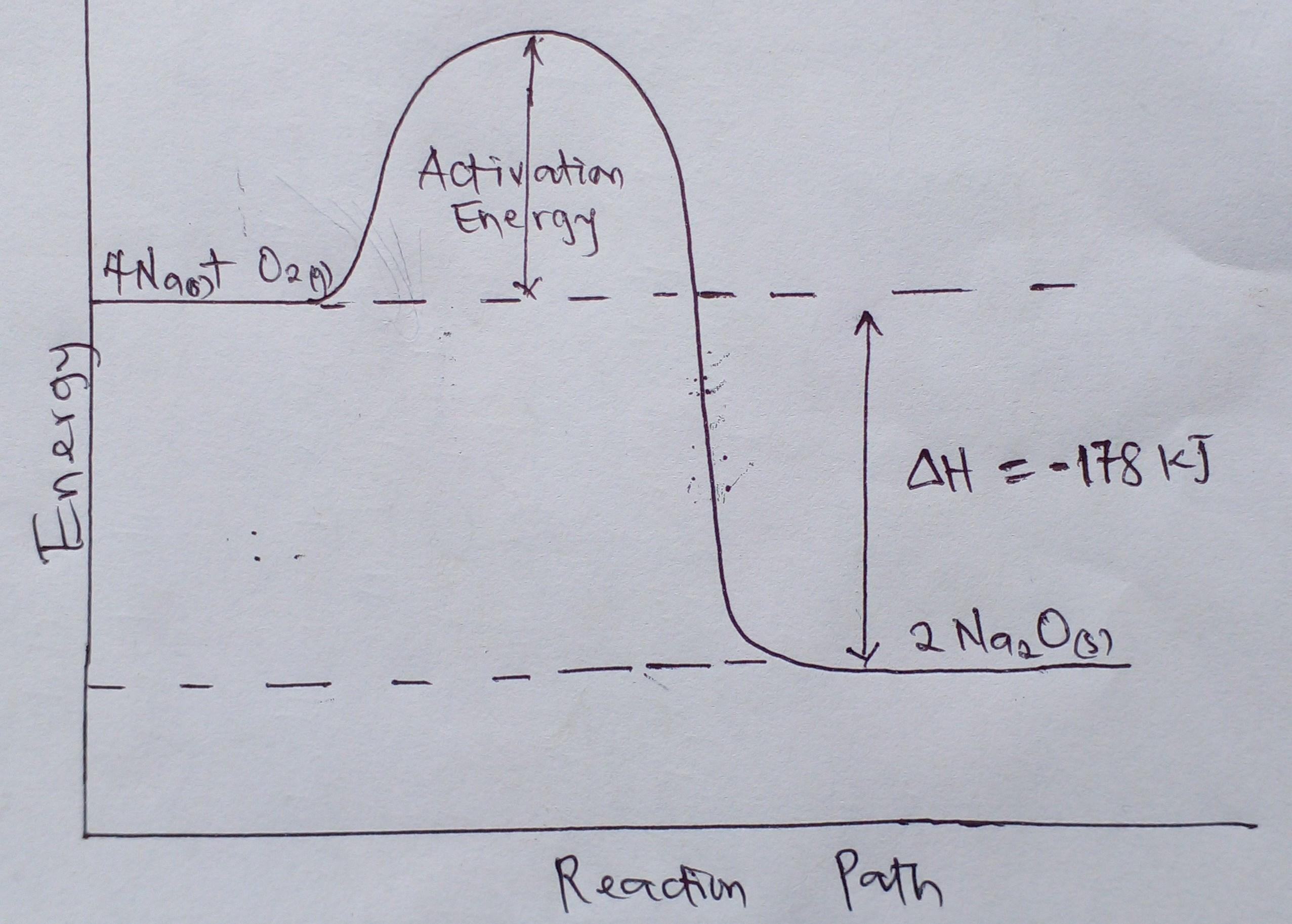
Draw a complete enthalpy diagram for the following reaction: 4Na(s) + O2(g) → 2Na2O(s). H=-178kJ
Answers
The enthalpy change of the given reaction is negative, so the reaction is an exothermic reaction.
What is enthalpy change of a reaction?Enthalpy change of a reaction is the energy changes that occur when reactant molecules form products.
Enthalpy is the heat content of a substance.
When enthalpy change is positive, the heat content of the product is greater than that of the reactant and the reaction is an endothermic reaction.
Whereas, when enthalpy change is negative, the heat content of the reactant is greater than that of the product and the reaction is an exothermic reaction.
Therefore, since the enthalpy change of the given reaction is negative, the reaction is an exothermic reaction.
Learn more about enthalpy changes at: https://brainly.com/question/11628413
#SPJ1