3. How many moles are in 100. grams of nitrogen gas (N2)?
I NEED HELO ASAP PLEASE
Answers
Answer:
12 moles
Explanation:
Related Questions
Tertiary or top consumers are animals at the top of the food web. They may feed off either primary or secondary consumers. What animals would fit into this classification?
Answers
Answer:
Coyotes, Bald Eagles, & Gray wolf.
write the conclusion of coin and feather experiment.
Answers
Answer: In a vacuum, the objects fall at the same rate independent of their respective masses. That means coin and feather both reach ground at same time in a vacuum. Larger objects experience more air resistance than smaller objects. Also, the faster an object falls, the more air resistance it encounters.
Explanation:
How many grams (g) are in 4.25 moles of Magnesium Chloride (MgCl2) ?
Answers
One mole of magnesium chloride has a mass of 95 g. Then the mass of 4.25 moles of magnesium chloride (MgCl₂) is 403.7 g.
What is magnesium chloride ?Magnesium chloride is an ionic compound with the chemical formula, MgCl₂. It formed through the donation of two valence electrons from the metal magnesium to the chlorine atoms.
The mass of one mole of a compound is called its molar mass. The number of moles of the compound is then the ratio of its given mass to the molar mass.
Atomic mass of Mg = 24 g/mol
atomic mass of Cl = 35.5 g/mol
mass of 2 Cl = 71 g
Then, molar mass of MgCl₂ = 24 + 71 g = 95 g/mol
One mole of MgCl₂ is 95 g. Then, mass of 4.25 moles of MgCl₂ is
4.95 × 95 = 403.7 g
Therefore, the mass of 4.95 moles of MgCl₂ is 403.7 g.
Find more on molar mass:
https://brainly.com/question/12127540
#SPJ2
EXERCISE 3: WHAT DOES pCO2 CHANGE? - When pCO
2
increases, the concentration of total CO
2
dissolved in water - When pCO
2
increases, the concentration of only CO
2
dissolved in water - When pCO
2
increases, the pH - Which form of dissolved CO
2
is most common in water? Ocean acidification is the decrease in pH due to increasing atmospheric CO
2
concentration.
2
. Choose the correct word option in the statements below: - An organism that needs CO
2
is likely to fare better / worse under ocean acidification. - An organism that needs HCO
3
- is likely to fare better/worse under ocean acidification. - An organism that needs CO
3
2−
is likely to fare better/worse under ocean acidification.
Answers
pCO2 is an important factor that affects various aspects of water chemistry and the impacts of ocean acidification. When pCO2 increases, the concentration of total CO2 dissolved in water also increases. This leads to changes in pH, which decreases due to increasing atmospheric CO2 concentration.
When pCO2 rises, the concentration of only CO2 dissolved in water increases. The dissolved CO2 forms carbonic acid, which contributes to the acidification of the ocean. This increase in CO2 affects the equilibrium between CO2, HCO3-, and CO3^2-, shifting it towards higher levels of dissolved CO2 and H+ ions, resulting in a lower pH.
In terms of the impacts of ocean acidification on different organisms, the effects can vary depending on their specific needs. An organism that requires CO2 is likely to fare better under ocean acidification since the increase in dissolved CO2 can provide them with a favorable environment. However, organisms that rely on HCO3- or CO3^2- may fare worse under ocean acidification, as the lower pH interferes with the availability of these carbonate ions, which are essential for shell formation and calcification in some marine organisms.
To know more about pCO2, click here, https://brainly.com/question/33500517
#SPJ11
Air pressure is caused by: gas particles striking the walls of a container or an object. b. gas particles are taking a test gas particles reaching 0 degrees Kelvin d. Air pressure has nothing to do with gas.
Answers
Answer:
D
Explanation:
Write the general formula of an alkane and use this to predict the 97th member
of the alkane series.
Answers
Explanation:
The general formula of an alkane is given as;
CₙH₂ₙ₊₂
n is the number of the member;
For the 97th member in the series;
n = 97;
2n + 2 = 2(97) + 2 = 196
So, the 97th member is;
C₉₇H₁₉₆
This is how to apply the formula of alkanes which are saturated hydrocarbons to find any member of the series.
can someone like help me pls ✨

Answers
Answer:
C
Explanation:
It is c because that goes into heating and cooling and if nobody is home you should definetly turn off heating and cooling. This should be the correct answer.
in a ______, substance have no definitie volume and particles move very quickly
Answers
Consider the statement, “In a chemical reaction, atoms are neither created nor destroyed, only rearranged.” Why is this a statement of the law of conservation of mass?
Answers
Answer: The law of conservation of mass is reffering to the fact that energy cannot be created or destroyed. So when you speak about atoms not being created or destroyed it is the same thing. Unless you're talking about an atomic bomb where the atoms are split.
In a chemical reaction, the total mass present is equal to the mass of all the atoms undergoing the reaction.
According to the law of conservation of mass, mass is neither created nor destroyed but can be converted from one form to another. In a chemical reaction, atoms are rearranged. However, the total number of atoms in the system remains constant.
So, the total mass present in the system is equal to the mass of all the atoms undergoing the reaction. Since atoms are only rearranged, no new atom is formed and no atom disappears in the reaction, the total mass of the system remains constant.
For this reason, the statement; “In a chemical reaction, atoms are neither created nor destroyed, only rearranged" is a statement of the law of conservation of mass.
Learn more: https://brainly.com/question/13383562
Find the amount of heat in joules required to raise the temperature of 28.0 grams of water from 30.0°C to 60.0°C.
Answers
Answer: 3510 J
Explanation:
q = m x Cs x delta T
m = 28.0 g
Cs = 4.18 J/g x ⁰C
delta T = Final temperature - Initial temperature = 60.0⁰C - 30.0⁰C = 30.0⁰C
q = m x Cs x delta T
q = 28.0 g x (4.18 J/g x ⁰C) x 30.0⁰C = 3511.2 J
3510 J for the correct number of significant figures.
Grams and ⁰C cancel and you are left with Joules.
What is an empirical formula?
Answers
Answer:
a formula giving the proportions of the elements present in a compound but not the actual numbers or arrangement of atoms.
Hope this helped!!!
Answer:
Empirical formulas show the simplest whole-number ratio of atoms in a compound
Explanation:
Balance the following redox reaction if it occurs in basic solution. What are the coefficients in front of Cr and Cl2 in the balanced reaction?
Cr(s) + Cl2(g) → Cr3+(aq) + Cl−(aq)
Cr = 2, Cl2 = 3
Cr = 2, Cl2 = 4
Cr = 1, Cl2 = 2
Cr = 2, Cl2 = 1
Cr = 3, Cl2 = 2
Answers
The correct answer for balanced reaction is : Cr = 1, Cl2 = 3
To balance the redox reaction in basic solution, we need to ensure that the number of electrons gained and lost are equal and that the charges are balanced. Here is the balanced equation:
Cr(s) + 3Cl2(g) + 6OH-(aq) → Cr(OH)3(s) + 6Cl-(aq)
In this balanced equation, the coefficients in front of Cr and Cl2 are:
Cr = 1, Cl2 = 3
The balanced equation shows that 1 mole of Cr reacts with 3 moles of Cl2 to form 1 mole of Cr(OH)3 and 6 moles of Cl-. The coefficients are chosen to balance the charges and the number of atoms on both sides of the equation.
Therefore, the correct answer is:
Cr = 1, Cl2 = 3
for more questions on balanced
https://brainly.com/question/11904811
#SPJ8
Sodium Tetraborate, a naturally occurring mineral, is crushed into Borax. This is an example of a ________change. *
Chemical Change
Synthetic Change
Physical Change
Please Help I will mark brainllest for the first answer. If you dont know the answer do not answer please.
Answers
Answer:
Chemical Change
Explanation:
Use the periodic table to answer the questions below.
Which diagram shows the correct electron
configuration for nitrogen (N)?
N N N1
1s
2s
2p
NN ILL
1s 2s
2p
N N
1s
2s
DONE✔
2p

Answers
The diagram that show the correct electron configuration is the second option. ⬆⬇1s, ⬆⬇2s, ⬆⬆⬆2p.
What is electronic configuration?The electronic configuration is the representation of electron present indifferent energy levels is an atom. The different type of orbitals are s, p, d, and f.
Thus, the correct option is 2, ⬆⬇1s, ⬆⬇2s, ⬆⬆⬆2p.
Learn more about electronic configuration
https://brainly.com/question/14283892
#SPJ1
4. When iron is oxidized, loses electrons, why does the electron come from the valence shell (45) instead of the highest energy shell (3d)?
Answers
Oxidization is the process of losing electrons. As iron is oxidized, it loses electrons from valence shell instead of highest energy shell because 4s orbital lies in the fourth shell, and 3d in the third shell and the 4s electrons being shielded from the nucleus by the inner 3d orbitals.
What is highest energy shell?The topmost shell has the utmost energy. So, the valence shell has the highest energy.
Energy is in the form of potential energy to allow the electrons to bind to the nucleus.
4s orbital lies in the fourth shell, and 3d in the third shell and the 4s electrons being shielded from the nucleus by the inner 3d orbitals.
Thus, iron is oxidized, it loses electrons from valence shell instead of highest energy shell.
For more details regarding energy shell, visit:
https://brainly.com/question/20861869
#SPJ2
There are many compounds composed of nitrogen and oxygen compare the formulas for nitrogen monoxide and nitrogen dioxide
Answers
The comparison of the formulas for nitrogen monoxide and nitrogen dioxide is: The formula for nitrogen monoxide (NO) is NO and the formula for nitrogen dioxide (NO2) is NO2.
What is their difference?Nitrogen monoxide (NO) and nitrogen dioxide (NO2) are both compounds made up of nitrogen and oxygen.
The difference between the two lies in the number of oxygen atoms present in each molecule.
NO contains one oxygen atom, while NO2 contains two oxygen atoms. NO is commonly known as nitric oxide and is a colorless and odorless gas that acts as a signaling molecule in the body.
NO2, on the other hand, is a reddish-brown gas that has a sharp, pungent odor and is a toxic air pollutant.
Read more about nitrogen and oxygen here:
https://brainly.com/question/9154002
#SPJ1
Write a balanced equation for the following nuclear decay reaction.
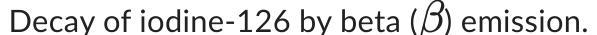
Answers
The balanced equation for the beta emission decay of iodine-126 is as follows:
Iodine-126 (126I) → Xenon-126 (126Xe) + Beta particle (β-)What is decay process in nuclear reaction?Decay process in nuclear reactions refers to the spontaneous transformation of an unstable nucleus into a more stable one, emitting one or more particles or radiation in the process.
The types of decay include
alpha decay, beta decay, and gamma decay.This process results in a decrease in the total number of protons and neutrons in the nucleus and a decrease in the total atomic number and mass number of the nucleus.
In the equation, the beta particle is an electron emitted from the nucleus during the decay process.
Learn more about beta emission at:
https://brainly.com/question/16409346
#SPJ1
label the ions so that the unit cell represents the structure of an inverse spinel, cofe2o4.
Answers
Eight tetrahedral sites are labeled with Fe³⁺ ions and four octahedral sites are labeled with Co²⁺ ions.
To represent the inverse spinel structure of CoFe₂O₄ in a unit cell, we need to label the ions based on their positions in the crystal structure.
In the inverse spinel structure, one-half of the octahedral sites are occupied by divalent metal ions, while one-half of the tetrahedral sites and the remaining octahedral sites are occupied by trivalent metal ions. The chemical formula of CoFe₂O₄ suggests that the cobalt (Co) ions occupy one-half of the octahedral sites, while the iron (Fe) ions occupy both the tetrahedral and the remaining half of the octahedral sites.
Therefore, we can label the ions in the unit cell of CoFe₂O₄ as follows:
Eight tetrahedral sites are labeled with Fe³⁺ ions.
Four octahedral sites are labeled with Co²⁺ ions.
Four octahedral sites are labeled with Fe³⁺ ions.
The unit cell of CoFe₂O₄ can be represented by the following diagram, where the small spheres represent Co²⁺ ions, and the large spheres represent Fe³⁺ ions:
Note that this is a simplified representation of the unit cell, as it only shows one layer of ions. In reality, the structure of CoFe₂O₄ is three-dimensional, and the unit cell contains many more ions.
The correct question is :
Label the ions so that the unit cell represents the structure of an inverse spinel, CoFe₂O₄. (Picture below)
To know more about tetrahedral follow the link:
https://brainly.com/question/14007686
#SPJ4


What is enthalpy change and volume change of mixing of two components forming an ideal solution?
Answers
Enthalpy change of mixing refers to the energy change during the formation of an ideal solution. Volume change of mixing relates to the change in volume resulting from the mixing process.
What is enthalpy ?Enthalpy is a thermodynamic property that represents the total heat content of a system at constant pressure. It encompasses both the internal energy of the system and the work done by or on the system. Enthalpy is denoted by the symbol "H" and is typically measured in units of energy, such as joules (J) or calories (cal). Enthalpy accounts for the energy transferred as heat during chemical reactions or phase changes. Enthalpy is crucial in studying and analyzing various phenomena, including chemical reactions, phase transitions, and energy transfers in thermodynamic systems.
Volume change of mixing, on the other hand, relates to the change in volume resulting from the mixing process. It accounts for the variation in molecular interactions and the resulting effects on the overall volume of the mixture compared to the volumes of the individual components.
To learn more about enthalpy
https://brainly.com/question/14047927
#SPJ4
"No matter what phase water
is in, the water molecules
stay the same; they just move
differently."
1: explain why this evidence matters
Answers
Answer:
Yes, water molecules remain the same despite the phase
Explanation:
When water is in the form of ice, it molecules remain the same only the distance between the molecule. This distance is higher than that of the inter molecular distance between liquid water molecules. Due to this reason ice is lighter than water.
Now in gaseous phase, the intermolecular distance increases thereby making it lighter than solid ice and liquid water.
By calculating ΔSuniv at each temperature, determine if the melting of 1 mole of NaCl(s ) is spontaneous at 500°C and at 700 °C. S°NaCl(s) = 72.11 J/mol K , S°NaCl(l) = 95.06 J/mol K ΔH°fusion = 27.95 kJ/mol
Answers
The melting of 1 mole of NaCl(s) is spontaneous at 500°C but not at 700°C.
To determine the spontaneity of the melting process at each temperature, we need to calculate the change in Gibbs free energy (ΔG) using the equation:
ΔG = ΔH - TΔS
S°NaCl(s) = 72.11 J/mol K
S°NaCl(l) = 95.06 J/mol K
ΔH°fusion = 27.95 kJ/mol
At 500°C (773 K):
ΔS = S°NaCl(l) - S°NaCl(s) = 95.06 J/mol K - 72.11 J/mol K = 22.95 J/mol K
ΔG = ΔH°fusion - TΔS = 27.95 kJ/mol - (773 K)(22.95 J/mol K) = 27.95 kJ/mol - 17.78 kJ/mol = 10.17 kJ/mol
Since ΔG is positive (10.17 kJ/mol > 0), the melting of NaCl(s) is non-spontaneous at 500°C.
At 700°C (973 K):
ΔS = S°NaCl(l) - S°NaCl(s) = 95.06 J/mol K - 72.11 J/mol K = 22.95 J/mol K
ΔG = ΔH°fusion - TΔS = 27.95 kJ/mol - (973 K)(22.95 J/mol K) = 27.95 kJ/mol - 22.33 kJ/mol = 5.62 kJ/mol
Since ΔG is still positive (5.62 kJ/mol > 0), the melting of NaCl(s) is also non-spontaneous at 700°C.
Therefore, the melting of 1 mole of NaCl(s) is spontaneous at 500°C but not at 700°C. The positive values of ΔG indicate that the process is non-spontaneous at both temperatures, meaning that energy input would be required for the solid NaCl to melt.
To know more about Gibbs free energy refer here:
https://brainly.com/question/29753420#
#SPJ11
16. Analysis of a sample of a covalent compound showed that it contained 14.4% hydrogen and 85.6% carbon by mass. What is the empirical formula for the compound?
Answers
Answer:a
Explanation:
The health care provider orders KCL 30 mEq. The medication is available in a unit dose package labeled: KCL 60 mEq/10 mL. The medicine cup is marked teaspoons. How many teaspoons will the nurse administer? tsp
Answers
the nurse will administer approximately 1 teaspoon (tsp) of the medication.1 teaspoon (tsp) is approximately equal to 5 mL.
How many teaspoons will the nurse administer?To determine the number of teaspoons the nurse should administer, we need to calculate the equivalent volume of 30 mEq of KCL using the provided concentration of 60 mEq/10 mL.
First, we'll find the ratio of milliequivalents (mEq) to milliliters (mL) in the given concentration:
60 mEq / 10 mL = 6 mEq/mL
Next, we can set up a proportion to find the volume (in mL) that corresponds to 30 mEq:
6 mEq/mL = 30 mEq / X mL
To solve for X, we can cross-multiply:
6X = 30 * 1
6X = 30
X = 30 / 6
X = 5 mL
Since the medication cup is marked in teaspoons, we need to convert 5 mL to teaspoons.
1 teaspoon (tsp) is approximately equal to 5 mL.
Therefore, the nurse will administer approximately 1 teaspoon (tsp) of the medication.
Learn more about measurement
brainly.com/question/28913275
#SPJ11
HELP!!!!! I WILL GIVE BRAINLYEST write a 250 word report on a extinct or endangered animal
Answers
Answer:
bro thats plagiarism
Explanation:
Which question would most likely be studied by a biologist?
A.
How can a drug that treats cancer be synthesized?
B.
How can cancer-causing substances be regulated?
C.
How much ultraviolet radiation passes through the atmosphere?
D.
What causes cancer cells to grow uncontrollably?
Answers
Answer:
The correct answer would be - D. What causes cancer cells to grow uncontrollably?
Explanation:
A Biologist is a person that studies living organisms and the study of living organisms and their process called biology. Biology is a very vast field that involves many branches such as zoology, botany, biochemistry, immunology, biophysics, anatomy, ecology and many more.
So, given all are can be an example of the study biology wanted to do, however, the question most likely studied by a biologist would be What causes cancer cells to grow uncontrollably as it deals with an organism and their biological process.
Melted wax has a temperature of 54 deg. Celsius. Physical or chemical property?
Answers
The melting of solid wax to form liquid wax and the evaporation of liquid wax to form wax vapor are physical changes. The burning of the wax vapor is a chemical change.
how long ago did the Atlantic Ocean separate Canada from Ireland....plz answer I have no idea lol
Answers
Answer:
Around 150 million years ago
Explanation:
The Atlantic ocean was formed during the Jurassic period
Answer:
200 millon years ago
Explanation: please give me brainliest
[cu2 ] concentration in a copper-silver electrochemical cell is 0.1m at 25 oc. if the total cell potential is 0.422v, what is the [ag ] concentration?
Answers
Choose the mathematical sentence that most accurately expresses the
information in the problem, and would be most helpful in solving it.
After taking 4 pieces of fruit out of a bag, 12 pieces of fruit remained. How
many pieces of fruit were in the bag to start?
A. 12 +/-4
B. 12 -4
C. 4+/- 12
D. 12-4-f
OE. +4=12
F. /-4-12
A compound formed with potassium and bromine that can detect blood in a stain by reacting with it to form distinctive crystals
Answers
Answer:
Potassium Bromide
Explanation:
not sure but i think thats it
a species of sea slug that emits a toxic chemical from its skin has a black, white, and gray body. a species of flatworm found in the same environment has very similar coloration and markings. which of the following best describes why a large population of these flatworms came to exist over time?
Answers
The flatworm and sea slug's comparable coloring and markings imply that both have evolved to have some type of warning coloration, often known as aposematism.
This indicates that they have evolved to use vivid or striking colors or patterns to alert potential predators to their toxicity.
large populationIn this instance, mimicking the deadly sea slug's coloring has probably evolved in flatworms as a type of defensive adaptation.
The flatworms may be able to fool predators into believing they are similarly toxic by resembling toxic sea slugs, which could reduce their risk of being attacked or eaten.
Natural selection would eventually favor people who had this protective adaption, resulting in a bigger population of flatworms with comparable color and other characteristics.
Consequently, the most likely explanation for the enormous population of flatworms with comparable coloring and patterns is that, via the process of natural selection, they have developed to resemble the coloring of the toxic sea slug as a type of protective adaptation.
learn more about the large population here
https://brainly.com/question/30363195
#SPJ1