1) Draw the other significant resonance contributor for the following compound; include lone pairs of electrons, formal charges, and hydrogen atoms. 2) Add curved arrows to both structures to show the delocalization of electron pairs.
Answers
Indicate the delocalization of electron pairs using curved arrows.
1) To draw the other significant resonance contributor for the compound, identify the regions with lone pairs of electrons, double bonds, or formal charges. Look for the movement of these electrons that could form a new, equivalent structure.
2) To show the delocalization of electron pairs, add curved arrows to both structures. The tail of the arrow should start from the electron pair (lone pair or double bond) and the head of the arrow should point towards the new location of that electron pair.
If a lone pair forms a double bond, the arrow will point to the bond location. If a double bond is broken, the arrow will point to the atom that gains a lone pair.
Remember to include hydrogen atoms, lone pairs of electrons, and formal charges in both resonance structures.
To learn more about hydrogen, refer below:
https://brainly.com/question/28937951
#SPJ11
Related Questions
Is the focal point in front or behind the mirror? Front?
Answers
The focal point of a mirror can be located either in front or behind the mirror depending on the type of mirror being used. Generally, there are two types of mirrors: convex and concave.
A convex mirror has a surface that curves outward, which means that the focal point is located behind the mirror. This type of mirror is commonly used in security mirrors or in car side-view mirrors.
On the other hand, a concave mirror has a surface that curves inward, which means that the focal point is located in front of the mirror. This type of mirror is commonly used in telescopes, microscopes, and dental mirrors.
It is important to note that the position of the focal point can affect how the image is formed by the mirror. A concave mirror, for example, can create a magnified or inverted image depending on where the object is placed in relation to the focal point.
In summary, the focal point of a mirror can be either in front or behind the mirror depending on the type of mirror being used. Understanding the properties of different types of mirrors can help us better understand how images are formed and how we can use mirrors in various applications.
To learn more about the focal point, refer:-
https://brainly.com/question/16188698
#SPJ11
what is the autoionization of water? autoionization of water is what is the autoionization of water?autoionization of water is a process where water acts as an acid and a base when it reacts with itself. a process where water acts as a base when it reacts with itself. a process where water acts as neither an acid nor a base when it reacts with itself. a process where water acts as an acid when it reacts with itself.
Answers
The autoionization of water is a unique property that describes the process where water molecules act as both an acid and a base when they react with each other.
This process is also known as self-ionization, where a small percentage of water molecules dissociate into ions, H+ and OH-, spontaneously. This happens due to the presence of a weak hydrogen bond between the hydrogen and oxygen atoms in water molecules. This reaction is essential for many chemical reactions that occur in aqueous solutions since it determines the concentration of H+ and OH- ions in the solution, which is crucial for pH calculation.
It is interesting to note that pure water at 25°C has an equal concentration of H+ and OH- ions, which is why it is considered neutral. Autoionization of water is a fundamental concept in chemistry that helps us understand the unique behavior of water as a universal solvent.
Learn more about autoionization here:
brainly.com/question/17518331
#SPJ11
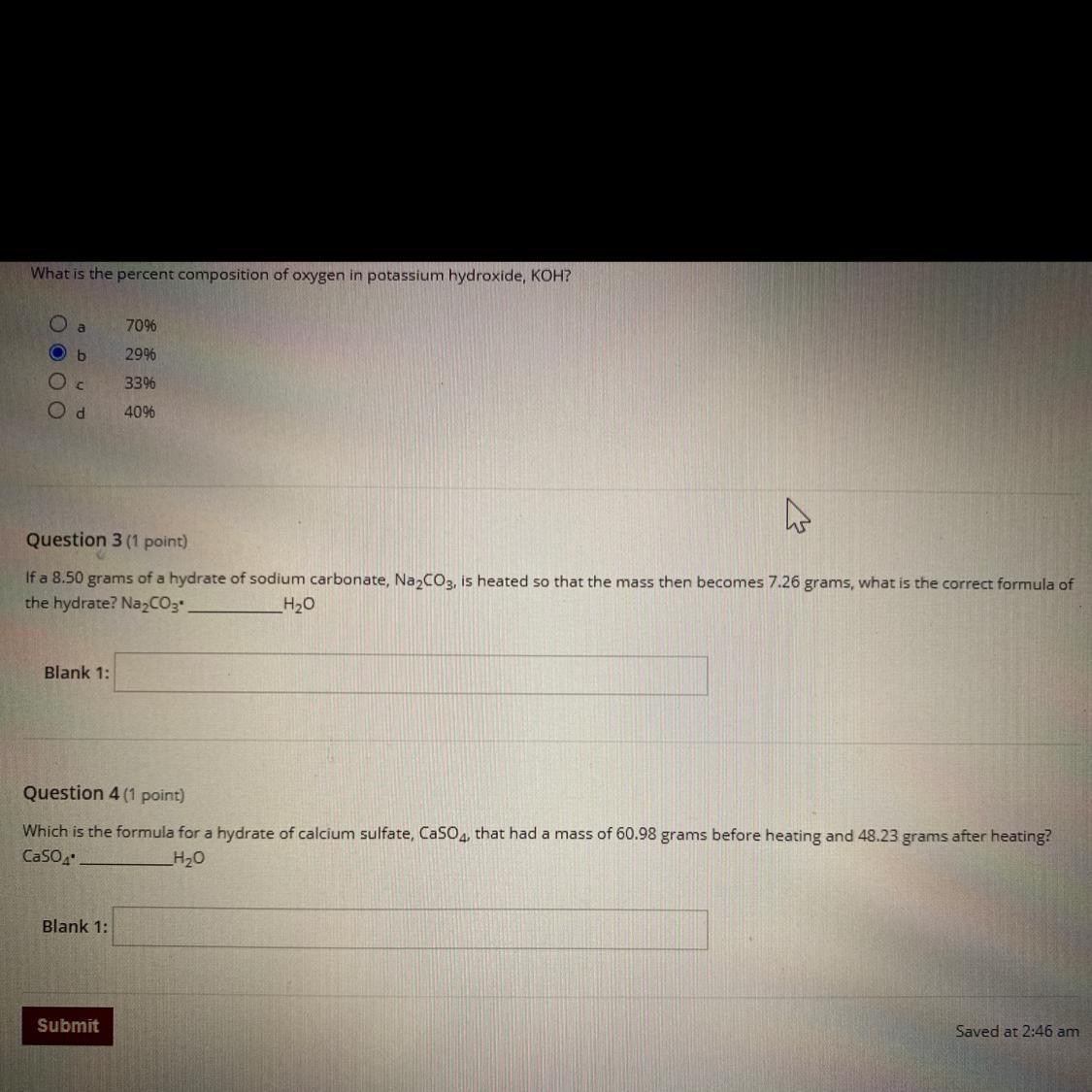
Question 3 (1 point)
If a 8.50 grams of a hydrate of sodium carbonate, Na2CO3, is heated so that the mass then becomes 7.26 grams, what is the correct formula of
the hydrate? Na2CO3 _H20
Blank 1:
Question 4 (1 point)
Which is the formula for a hydrate of calcium sulfate, CaSO4, that had a mass of 60.98 grams before heating and 48.23 grams after heating?
CaSO4
_H20
Blank 1:
Answers
Answer:
1. Formulae of hydrate is Na₂CO₃.10H₂O
2. Formulae of hydrate is CaSO₄.2H₂O
Explanation:
Q1. Mass of hydrated salt = 8.50 g mass
mass of anhydrous salt = 7.26 grams
mass of water lost = 1.24
Formula of hydrated salt = Na₂CO₃.xH₂O
Formula of anhydrous salt = Na₂CO₃
Molar mass of anhydrous salt is obtained as below where Na = 23, C = 12, O = 16, H = 1
Molar mass of Na₂CO₃ = (23 *2 + 12 + 16 * 3) = 106 g
molar mass of water = (1 *2 + 16) = 18 g
Mole ratio of Na₂CO₃ to H₂O = 7.26/106 : 1.24/18
Mole ratio of Na₂CO₃ to H₂O = 0.0068 : 0.068
Mole ratio of Na₂CO₃ to H₂O = 1 :10
Therefore, formulae of hydrate is Na₂CO₃.10H₂O
Q2. Mass of hydrated salt = 60.98 g mass
mass of anhydrous salt = 48.23 grams
mass of water lost = 12.75
Formula of hydrated salt = CaSO₄.xH₂O
Formula of anhydrous salt = CaSO₄
Molar mass of anhydrous salt is obtained as below where Ca = 40, S = 32, O = 16, H = 1
Molar mass of CaSO₄= (40 + 32 + 16 * 4) = 136 g
molar mass of water = (1 *2 + 16) = 18 g
Mole ratio of CaSO₄ to H₂O = 48.23/136 : 12.75/18
Mole ratio of CaSO₄ to H₂O = 0.35 : 0.70
Mole ratio of CaSO₄ to H₂O = 1 : 2
Therefore, formulae of hydrate is CaSO₄.2H₂O
Explanation:
Question 3:
Mass of anhydrous compound = 7.26g
Mass of water = 8.50 - 7.26 = 1.24 g
Percentage of water of crystallization in the compound is = (1.24 / 8.50) * 100 = 14.59%
The mass ratio of Na2CO3 : H2O = 7.26 : 1.24
Using mole = mass / molar mass;
The mole ratio of Na2CO3 : H2O = 7.26 /105.99 : 1.24 / 18 = 0.069 : 0.069 or 1 : 1
The formular is; Na2CO3.H2O
Question 4:
Mass of anhydrous compound = 48.23 g
Mass of water = 60.98 - 48.23 = 12.75 g
Percentage of water of crystallization in the compound is = (12.75 / 60.98) * 100 = 20.91%
The mass ratio of CaSO4 : H2O = 48.23 : 12.75
Using mole = mass / molar mass;
The mole ratio of CaSO4 : H2O = 48.23 / 136.14 : 12.75 / 18 = 0.3543 : 0.708 which is 1 : 2
The formular is; CaSO4.2H2O
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
Why is reduced precipitation, rather than drought, the leading cause of limited water availability?
A)
Reduced precipitation is the cause of drought.
B)
Drought only impacts the supplies of groundwater.
C)
Water availability depends upon drought conditions.
D)
Drought only affects areas with vegetation.
E)
Precipitation limits the amount of water in many environments.
Answers
Answer:
A. Reduced precipitation is the cause of drought.
Explanation:
Reduced precipitation begins to insufficient water availability because water catch basins or tanks tend to dry out. When it drains out, people tend to start working and drying out the backup water sources and stored ones. Until such time, that the reservoirs of water will completely be dried out. And there will be no more water for them to use and there will be a drought.
hope this helps
Answer:
Option A
Explanation:
Precipitation generally refers to the source of water like rain ,snow If precipitation decreases the rain water coming decreasesWhich decreases the water level on earthThat's the main reason behind droughtHence option A is correctFILL IN THE BLANK. ___ occurs when an electron in an atom jumps from a lower energy orbital to a higher energy orbital.
Answers
Radiation occurs when an electron in an atom jumps from a lower energy orbital to a higher energy orbital.
Define electrons.
The elementary electric charge of the electron is a negative one, making it a subatomic particle. Due to their lack of known components or substructure, electrons, which are part of the first generation of the lepton particle family, are typically regarded to be elementary particles.
Energy released by matter as rays or swift particles is known as radiation. Atoms make up all physical matter. The nucleus of an atom includes tiny particles called protons and neutrons, and the outer shell of the atom is made up of other particles called electrons.
An electron loses a significant portion of its energy through a radiative nuclear interaction at extremely high speeds.
To learn more about radiation use:
https://brainly.com/question/31285748
#SPJ4
Laughing gas is commonly used in surgery and dentistry for its anesthetic and analgesic effects. It is known as laughing gas because it creates a strong sense of happiness when patients inhale it. What is the iupac name of the compound n2o?.
Answers
The IUPAC name of the compound N₂O, commonly known as laughing gas, is dinitrogen monoxide.
Laughing gas, or dinitrogen monoxide (N₂O), is a colorless, non-flammable gas often used in surgery and dentistry for its anesthetic and analgesic effects. When inhaled, it induces a strong sense of happiness, hence the name "laughing gas."
In the IUPAC naming system, the two nitrogen atoms are denoted by the prefix "di-" while the single oxygen atom is indicated by the prefix "mono-." Thus, combining these prefixes with the element names results in the IUPAC name "dinitrogen monoxide."
To know more about IUPAC name click on below link:
https://brainly.com/question/16631447#
#SPJ11
2- Bromo – 2 – methyl propane is :
(a) Primary alkyl halide
(b) Secondary alkyl Halide
(c) Tertiary alkyl amine
(d) Tertiary alkyl Halide
Answers
Answer:
(B) SECONDARY ALKYL HALIDE
NEED HELP ASAP 7th grade science btw pls

Answers
Answer: the answer is A or Tributaries, River system and rivers
Explanation: Its A i had the same question last year for my 10th grade assignment
How many nodes are present in Y5 of 1,3,5,7,9-decapentaene? A. 2 B. 3 C. 4 D. 5
Answers
The number of nodes present in Y5 of 1,3,5,7,9-decapentaene is 4, so the correct option is C.
In a molecular orbital diagram, the number of nodes can be determined by the molecular orbital's subscript number (Yn).
The formula for finding the number of nodes is n-1, where n is the subscript. In this case, for Y5, the formula would be 5-1 = 4.
Therefore, there are a total of four nodes present in Y5 of 1,3,5,7,9-decapentaene.
In summary, the answer to your question is C and there are four nodes present in Y5 of 1,3,5,7,9-decapentaene due to the presence of four carbon-carbon double bonds.
Learn more about decapentaene click here:
https://brainly.com/question/475709
#SPJ11
Please help with this:
Typically, hard water used in a lab class would have been prepared by adding 1 gram of
magnesium sulfate per liter of distilled water. Magnesium sulfate contains 20.2%
magnesium ions by mass. What is its hardness in grains per gallon (GPG)? (One GPG
equals 17.1 mg/L.)
Answers
One grain of calcium carbonate, or 64.8 milligrams, is dissolved in one US gallon of water to represent one grain per gallon (gpg), a measure of water hardness (3.785412 L).
What is Water Hardness Measurement Scales?Understanding your test findings necessitates familiarity with the many water hardness testing scales that are employed. The majority of results are provided as a number that indicates the amount of calcium carbonate or calcium carbonate equivalents present in a specific unit of water. Depending on the measurement method, this value may be given in grains per gallon (gpg), parts per million (ppm), or milligrammes per litre (mg/L).Per Gallon of Grains Measurement of water hardnessThe hardness scale, expressed in gpg of calcium carbonate, can be seen as follows, according to the Water Quality Association:Soft is defined as gpg less than 1.An intermediate level of difficulty is between 1 and 3.5 gpg.The category of fairly challenging ranges from 3.5 to 7 gpg.The hard range is between 7 and 10.5 gpg.It's regarded quite difficult to get above 10.5 gpg.To Learn more About grain per gallon refer to:
https://brainly.com/question/2350304
#SPJ1
How many Joules of energy are there in one photon of orange light whose wavelength is 630nm?
Answers
The energy are there in one photon of orange light whose wavelength is 630nm is \(3.15401\times10^{-19}\) Joules.
W=c/v, c=speed of light, v=frequency
\(6.3 \times 10^-7=3 \times 10^8/v\)
\(v=3 \times 10^8 / 6.3 \times 10^-7\)
\(v=4.76 X 10^14 Hz\)-frequency of the yellow light.
E=hv, h=Planck's constant,
\(E=(4.76 \times 10^14)Hz \times 6.62607×10^-34 J s\)
\(E=3.15401\times10^{-19}\) Joules- the energy of a single photon of yellow light.
What is Planck's constant?Planck's constant or Planck's constant, is a fundamental physical constant of quantum mechanics. The constant gives the ratio of the energy of the photon to its frequency, and for mass-energy equivalent, the ratio of mass to frequency.
In quantum mechanics, energy is exchanged and absorbed in certain amounts called quanta. The Planck constant is a number that defines the amount of energy in these quantities and expresses how small things can be. Learn more about Planck's constant in this infographic.
To learn more about Planck's constant, refer;
https://brainly.com/question/10700482
#SPJ10
Researchers can use solvents to extract, or break apart, components of a cell or tissue to separate and/or isolate components. If you extracted some cells with a non-polar organic solvent, what would the solvent contain the MOST of at the end of the process
Answers
The cell membrane which is more fat permeable will be present at the end of the process.
Solvent extraction is a very important method if separating mixtures. The principle of solvent extraction is based on the idea of like dissolves like. A substance dissolves in the component of the system in which it is most soluble.
If a cell extract is dissolved in a nonpolar solvent, the cell membrane which is mostly permeable to nonpolar molecules will dissolve most in the solvent.
Learn more:https://brainly.com/question/14396802
Answer:
A. chlorophyll
Explanation:
Plants use chlorophyll the most & I took the test.
Which best represents a heterogeneous mixture?
salt dissolved in water
hydrochloric acid
a mixture of oxygen gas and nitrogen gas
sand at the beach
ANSWER ASAP
Answers
explain why zn reacts more slowly with dilute hydrochloric acid then with concentrated hydrochloric acid
Answers
Answer:
When the concentration is higher, more hydrogen ions are near the Zn atoms at any given time. This allows for more Zn atoms to be ionized and dissolved into the solution per second.
Explanation:
Describe how you would prepare a pure dry sample of lead(II) sulfate crystals starting from solutions of lead(II) nitrate and sodium sulfate.
Include a series of key steps in your answer.
Answers
Answer:
Method: Measure out 25 cm3 of 0.5 mol dm3 lead(II)nitrate solution and add it to a small beaker. Measure out 25 cm3 of 0.5 mol dm3 of potassium sulfate add it to the beaker and mix together using a stirring rod.
What is one characteristic of a biofilm?
Answers
what is the answer to 2.5 * 22.5
Answers
write the empirical formula for at least four ionic compounds that could be formed from the following ions: , , ,
Answers
The empirical formula for an ionic compound comprising at least four ions, including \(Fe^{3+}\), \(NO^{3-}\), \(NH^{4+}\), and \(CN^{-}\), are:
\(Fe(CN)_6^{3-}\) , \(Fe(NO3)_3\) , \(NH_4NO_3\) , \((NH_4)_2Fe(CN)_6\)
\(Fe^{3+}\): \(Fe(CN)_6^{3-}\) is the empirical formula for this ionic compound. It is made up of cyanide ions \(CN^{-}\)and iron(III) ions \(Fe^{3+}\).
\(NO^{3-}\): The empirical formula for this ionic molecule \(Fe(NO3)_3\) is \(NO^{3-}\) and \(Fe^{3+}\). It is created when nitrate ions and iron(III) ions \(Fe^{3+}\) combine.
\(NH^{4+}\) : The empirical formula for this ionic molecule is \(NH_4NO_3\). It is made up of nitrate and ammonium ions.
\(CN^{-}\) : This ionic compound, which has the empirical formula \((NH_4)_2Fe(CN)_6\), contains the ions ammonium, iron(III) , and cyanide.
The relative ratios of the ions involved in the creation of each compound are shown by these empirical formulas, which give a clear picture of the compounds.
To know more about empirical formula,
https://brainly.com/question/29416729
The complete question is-
Write the empirical formula for at least four ionic compounds that could be formed from the following ions:
\(Fe^{3+}\) , \(CN^{-}\) , \(NO^{3-}\) , \(NH^{4+}\).
#SPJ4
how many grams of sulfur can be formed from 108g of sulfur dioxide?
Answers
Answer:
1.99 grams
Explanation:
hope this helps
How are the number of protons and neutrons represented in a periodic table?
Answers
Answer:
The atomic number or proton number (symbol Z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The atomic number uniquely identifies a chemical element.
Explanation:
How many of the following are WEAK acids?
HNO2 HF HNO3 H2PO4^-
a. 0
b. 1
c. 4
d. 2
e. 3
Answers
The weak acids are HNO₂ and HF. Option D is correct.
HNO₂ (nitrous acid) and HF (hydrofluoric acid) are considered weak acids because they only partially dissociate in water, resulting in a relatively low concentration of H⁺ ions in solution. On the other hand, HNO₃ (nitric acid) and H₂PO₄⁻ (dihydrogen phosphate) are strong acids, which fully dissociate in water, producing a high concentration of H⁺ ions.
On the other hand, HNO₃ (nitric acid) and H₂PO₄⁻ (dihydrogen phosphate) are both strong acids;
HNO₃ is a strong acid that fully dissociates in water, resulting in a high concentration of H⁺ ions.
H₂PO₄⁻ is a weak acid in its conjugate acid form (dihydrogen phosphate), but as H₂PO₄⁻, it acts as a weak base rather than a weak acid.
Hence, D. is the correct option.
To know more about weak acids here
https://brainly.com/question/32730049
#SPJ4
Wildflowers bloom earlier than large trees. This helps wildflowers compete for _____. water, light, food, or air
Answers
Answer: Light
Explanation:
The environment abiotic factors like sunlight, water, air, and soil are important for the growth of the plants. A wildflowers is a plant that grows in wild. It was not intentionally seeded or planted.
The light is one of the important environmental factor. As the wildflowers are blooming earlier than the large trees. Thus the wildflowers are receiving the light earlier than large trees.
A1 L sample from Tempe Town lake has a pH of 3. 57. Exhaust from cars on nearby highways
often mixes with rain to form HNO3. Use this information to answer the following questions.
1. What is the concentration of H+ ions in the sample?
2. If it took 80 ml of 1 M NaOH to neutralize 250 ml of the acidic solution, what is the
molarity of HNO3 in the sample?
3. How many moles of HNO3 are in the
solution?
Answers
Answer:
see explanations
Explanation:
1. pH = -log [H+]
so [H+] = 10^(-pH) = 10^(-3.57) = 2.7 x 10^(-4) M
2. neutralize implies mol acid = mol base
so [HNO3] = (1 M) * (0.080 L) / (0.250 L) = 0.32 M HNO3
3. mol HNO3 = molarity of HNO3 * volume of HNO3
= 0.32 M HNO3 * 0.250 L HNO3 = 0.080 mol HNO3
A reaction that releases energy is called endothermic .
True or False
Answers
Answer: False
Explanation: Endothermic means it absorbs heat energy.
1. Which term identifies a factor that will shift a
chemical equilibrium?
A) atomic radius
B) catalyst
C) decay mode
D) temperature
Answers
Answer:
D) Temperature,
Explanation:
You have a 5 mL sample of a protein in 0.5 M NaCl. You place the protein/salt sample inside dialysis tubing (see Fig. 2-14) and place the bag in a large beaker of distilled water. If your goal is to remove as much NaCl from the sample as possible, which would be more eff ective: (1) placing the dialysis bag in 4 L of distilled water for 12 h, or (2) placing the bag in 1 L of distilled water for 6 h and then in another 1 L of fresh distilled water for another 6 h
Answers
Placing the bag in 1 L of distilled water for 6 h and then in another 1 L of fresh distilled water for another 6 h would be more effective.
Dialysis tubing is a semi-permeable membrane used to remove small molecules from larger molecules like proteins. Dialysis is a critical process in protein purification, as it enables the removal of small molecules like salts from the protein solution.
The movement of molecules across the membrane occurs through a process known as diffusion. In the given scenario, the aim is to remove as much NaCl as possible from the protein sample. The rate of salt removal from the protein solution depends on the concentration gradient of salt inside and outside the dialysis tubing. The faster the movement of water molecules into the dialysis bag, the faster the salt removal process. Therefore, we need to create a higher concentration gradient of salt for faster diffusion.
Both procedures can remove NaCl, but the rate of removal will vary with the concentration gradient. The concentration gradient is higher when placing the bag in 1 L of distilled water for 6 h and then in another 1 L of fresh distilled water for another 6 h, and it is lower when placing the dialysis bag in 4 L of distilled water for 12 h.
When you put the dialysis bag in 4 L of distilled water for 12 hours, the concentration gradient is reduced. It would be best to place the dialysis bag in 1 L of distilled water for 6 h and then in another 1 L of fresh distilled water for another 6 h to achieve a higher concentration gradient of salt inside and outside the bag. This will make the salt removal process more effective.
to know more about Dialysis here:
brainly.com/question/31029018
#SPJ11
How does the suns energy contribute to the movement of water in the water cycle?
Answers
Answer:Evaporation
Explanation:
The suns heat speeds the movement of water molecules, until the molecules move fast enough that they jolt out the surface of the water individually. Overtime the water gradually evaporates into water vapor.
a sample of he gas at 3.0 l and 5.6 atm was combined with a sample of ne gas at 4.5 l and 3.6 atm in a single flask of volume 9.0 l at constant temperature. what is the total pressure in the flask assuming that the initial pressure was 0 atm?
Answers
P = 3.67 atm is the total pressure in the flask assuming that the initial pressure was 0 atm .
What is Ideal gas law ?
The ideal gas law (also called the perfect gas law), the relationship between the pressure P, volume V, and temperature T of a gas in the boundary region between low pressure and high temperature. Gas molecules move almost independently. each other .
PV = nRT, where n is the number of moles of gas and R is the universal (or perfect) gas constant, 8.31446261815324 joules/kelvin/mol (the universal gas constant is defined as Avogadro's number NA multiplied by Boltzmann's constant k) . ) In the International System of Units, energy is measured in joules, volume in cubic meters (m3), force in newtons (N), and pressure in pascals (Pa). where 1 Pa = 1 N/m2. A force of 1 Newton moving a distance of 1 meter does 1 Joule of work. Therefore, the product of both PV and nRT has the dimension of work (energy).
For He: PV = nRT and n = PV/RT
n = (5.6 atm)(3.0 L)/(0.0821 Latm/Kmol)(298K) = 0.687 moles He
For Ne: PV = nRT and n = PV/RT
n = (3.6 atm)(4.5 L)/(0.0821 Latm/Kmol)(298K) = 0.662 moles Ne
Total moles = 0.687 + 0.662 = 1.349 moles
PV = nRT
P = nRT/V
P = (1.349 mol)(0.0821 Latm/Kmol)(298 K)/9.0 L
P = 3.67 atm
To learn more about Ideal gas law , click the link below ;
https://brainly.com/question/21912477
#SPJ4
In winter, soap does not dissolve properly in water.
Answers
Answer:
because of nothing...............................